Th17/Treg cell balance in patients with papillary thyroid carcinoma: a new potential biomarker and therapeutic target
- PMID: 39534095
- PMCID: PMC11554530
- DOI: 10.3389/fonc.2024.1325575
Th17/Treg cell balance in patients with papillary thyroid carcinoma: a new potential biomarker and therapeutic target
Abstract
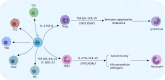
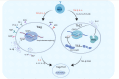
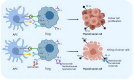
Papillary thyroid carcinoma (PTC) is the most common subtype of thyroid carcinoma. The most effective treatment for PTC is surgical resection, and patients who undergo surgery have good survival outcomes, but some patients have distant metastasis or even multiorgan metastases at the time of initial diagnosis. Distant metastasis is associated with poorer prognosis and a higher mortality rate. Helper T lymphocyte 17 (Th17) cells and regulatory T lymphocytes (Tregs) play different roles in PTC, and the Th17/Treg balance is closely related to the progression of PTC. Th17 cells play anticancer roles, whereas Tregs play cancer-promoting roles. A Th17/Treg imbalance promotes tumor progression and accelerates invasive behaviors such as tumor metastasis. Th17/Treg homeostasis can be regulated by the TGF-β/IL-2 and IL-6 cytokine axes. Immune checkpoint inhibitors contribute to Treg/Th17 cell homeostasis. For PTC, monoclonal antibodies against CTLA-4, PD-1 and PD-L1 inhibit the activation of Tregs, reversing the Th17/Treg cell imbalance and providing a new option for the prevention and treatment of PTC. This article reviews the role of Tregs and Th17 cells in PTC and their potential targets, aiming to provide better treatment options for PTC.
Keywords: Th17; Th17/Treg homeostasis; Treg; checkpoint blockade; papillary thyroid carcinoma (PTC).
Copyright © 2024 Huo, Adeerjiang, Abulitipu, Khan, Li, Zhang, Tian, Jiang, Xu, Chao, Yang, Zhang and Du.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the american thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. (2015) 2016:26(1). doi: 10.1089/thy.2015.0020 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

