Open-Label Phase 1/2 Study of Daratumumab-Based Desensitization Before Kidney Transplantation
- PMID: 39534185
- PMCID: PMC11551132
- DOI: 10.1016/j.ekir.2024.08.020
Open-Label Phase 1/2 Study of Daratumumab-Based Desensitization Before Kidney Transplantation
Abstract
Introduction: The safety and benefit of the anti-CD38 monoclonal antibody daratumumab, which induces lysis of antibody-producing plasma cells in sensitized patients prior to kidney transplantation, remain to be determined.
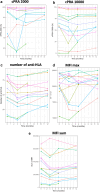
Methods: A 2-phase (1 and 2), monocentric open-label study was conducted to evaluate the month 6 (M6) safety and efficacy of daratumumab in kidney transplant candidates with calculated panel reactive antibody (cPRA) > 95%. In the first (safety) phase, we used 4-weekly escalating doses of daratumumab. Phase 2 tested desensitization with 8 weekly infusions of 16 mg/kg daratumumab. cPRA 10,000 was calculated considering only human leukocyte antigen (HLA) antibodies with mean fluorescence intensity (MFI) of > 10,000.
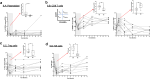
Results: Nine patients were enrolled in phase 1 and 14 in phase 2. Safety analysis showed 4 serious non-treatment-emergent adverse events (non-TEAEs), 36 mild TEAEs, mostly infusion-related reactions, grade 1 and 2 (causing 2 temporary drug discontinuations), but no serious TEAEs. Significant reductions in anti-HLA antibodies were observed at month 3 (M3), with cPRA 10,000 (P = 0.003), number of anti-HLA (P < 0.001), maximum MFI (MFI max) (P = 0.053), and the sum of MFI (MFI sum) (P < 0.001), with complete return to baseline levels at month 12 (M12). At M6, 46.15% (19.22%-74.87%) and 76.92% (46.19%-94.96%) of patients showed sustained response (1% decrease in cPRA) for cPRA 2000 and 10,000, respectively. At month 1 (M1), immune cells (T-reg, CD8 + TEMRA, CD19 + CD138 + B cells, and NK cells) significantly decreased. At M3, other antibodies decreased significantly, but returned to baseline levels at M12, except for gamma globulins, without any infectious complications.
Conclusion: The first use of daratumumab in desensitization demonstrated infusion-related adverse (AEs) events and rapid, albeit transient, reductions in anti-HLA antibodies, with less than 40% of durable responders, limiting its potential clinical use.
Keywords: lymphocytes; pharmacokinetics; translational; transplantation.
© 2024 International Society of Nephrology. Published by Elsevier Inc.
Figures


References
LinkOut - more resources
Full Text Sources
Research Materials

