Durable left ventricular assist devices following temporary circulatory support on a microaxial flow pump with and without extracorporeal life support
- PMID: 39534325
- PMCID: PMC11551302
- DOI: 10.1016/j.xjon.2024.06.021
Durable left ventricular assist devices following temporary circulatory support on a microaxial flow pump with and without extracorporeal life support
Abstract
Background: Circulatory support with a catheter-based microaxial flow pump (mAFP) plays a major role in the treatment of severe cardiogenic shock. In most patients who fail to recover while on temporary mechanical circulatory support (tMCS) and who are not eligible for heart transplantation, durable left ventricular assist device (dLVAD) implantation is usually considered a reliable option. This study aimed to describe the outcome of dLVAD therapy following mAFP support and to identify predictors of mortality.
Methods: This was a retrospective analysis of data from a multicenter registry on patients who underwent dLVAD implantation following tMCS with a mAFP between January 2017 and October 2022 (n = 332) from 19 European centers.
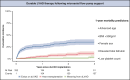
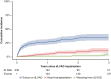
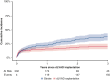
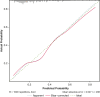
Results: Patients were supported with an Impella 5.5 (n = 92), 5.0 (n = 153) or CP (n = 87) and were transitioned to a HeartWare HVAD (n = 128) or Heartmate 3 (n = 204) during the same period. One hundred and twenty-five patients (39.2%) also required extracorporeal life support before and/or during mAFP therapy. The 30-day and 1-year survival were 87.8% and 71.1%, respectively. The following risk factors for 1-year mortality were identified: age (odds ratio [OR], 1.02), specifically age over 55 years (OR, 1.09), body mass index >30 kg/m2 (OR, 2.2), female sex (OR for male sex, 0.43), elevated total bilirubin (OR, 1.12), and low platelet count (OR, 0.996).
Conclusions: Based on the identified risk factors, a risk score for estimating 1-year mortality was calculated to optimize patient selection for dLVAD implantation.
Keywords: Impella; LVAD; cardiogenic shock; mechanical circulatory support; outcome.
© 2024 The Author(s).
Conflict of interest statement
M.O. is a member of the advisory board for A biomed. All other authors reported no conflicts of interest. The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Figures

References
-
- McDonagh T.A., Metra M., Adamo M., et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–3726. - PubMed
-
- Saeed D., Potapov E., Loforte A., et al. Transition from temporary to durable circulatory support systems. J Am Coll Cardiol. 2020;76:2956–2964. - PubMed
-
- Eulert-Grehn J.J., Starck C., Kempfert J., Falk V., Potapov E. ECMELLA 2.0: single arterial access technique for a staged approach in cardiogenic shock. Ann Thorac Surg. 2021;111:e135–e137. - PubMed
-
- Tsyganenko D., Gromann T.W., Schoenrath F., et al. Predictors of mid-term outcomes in patients undergoing implantation of a ventricular assist device directly after extracorporeal life support. Eur J Cardiothorac Surg. 2019;55:773–779. - PubMed
-
- Bertoldi L.F., Pappalardo F., Lubos E., et al. Bridging INTERMACS 1 patients from VA-ECMO to LVAD via Impella 5.0: De-escalate and ambulate. J Crit Care. 2020;57:259–263. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

