Combination of artificial intelligence endoscopic diagnosis and Kimura-Takemoto classification determined by endoscopic experts may effectively evaluate the stratification of gastric atrophy in post-eradication status
- PMID: 39534404
- PMCID: PMC11555298
- DOI: 10.1002/deo2.70029
Combination of artificial intelligence endoscopic diagnosis and Kimura-Takemoto classification determined by endoscopic experts may effectively evaluate the stratification of gastric atrophy in post-eradication status
Abstract
Background: Since it is difficult for expert endoscopists to diagnose early gastric cancer in post-eradication status, it may be critical to evaluate the stratification of high-risk groups using the advance of gastric atrophy or intestinal metaplasia. We tried to determine whether the combination of endoscopic artificial intelligence (AI) diagnosis for the evaluation of gastric atrophy could be a useful tool in both pre- and post-eradication status.
Methods: 270 Helicobacter pylori-positive outpatients (Study I) were enrolled and Study II was planned to compare patients (n = 72) with pre-eradication therapy with post-eradication therapy. Assessment of endoscopic appearance was evaluated by the Kyoto classification and Kimura-Takemoto classification. The trained neural network generated a continuous number between 0 and 1 for gastric atrophy.
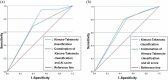
Results: There were significant associations between the severity of gastric atrophy determined by AI endoscopic diagnosis and not having a regular arrangement of collecting venules in angle, visibility of vascular pattern, and mucus using Kyoto classification in H. pylori-positive gastritis. There were significant differences (p = 0.037 and p = 0.014) in the severity of gastric atrophy between the high-risk group and low-risk group based on the combination of Kimura-Takemoto classification and endoscopic AI diagnosis in pre- and post-eradication status. The area under the curve values of the severity of gastric atrophy (0.674) determined by the combination of Kimura-Takemoto classification and gastric atrophy determined by AI diagnosis was higher than that determined by Kimura-Takemoto classification alone in post-eradication status.
Conclusion: A combination of gastric atrophy determined by AI endoscopic diagnosis and Kimura-Takemoto classification may be a useful tool for the prediction of high-risk groups in post-eradication status.
Keywords: Helicobacter pylori; Kyoto Classification; artificial intelligence; atrophic gastritis; gastric cancer.
© 2024 The Author(s). DEN Open published by John Wiley & Sons Australia, Ltd on behalf of Japan Gastroenterological Endoscopy Society.
Conflict of interest statement
None.
Figures

Similar articles
-
Determination of gastric atrophy with artificial intelligence compared to the assessments of the modified Kyoto and OLGA classifications.JGH Open. 2022 Aug 26;6(10):704-710. doi: 10.1002/jgh3.12810. eCollection 2022 Oct. JGH Open. 2022. PMID: 36262541 Free PMC article.
-
Endoscopic severe mucosal atrophy indicates the presence of gastric cancer after Helicobacter pylori eradication -analysis based on the Kyoto classification.BMC Gastroenterol. 2020 Jul 20;20(1):232. doi: 10.1186/s12876-020-01375-z. BMC Gastroenterol. 2020. PMID: 32689949 Free PMC article.
-
Kyoto classification risk scoring system and endoscopic grading of gastric intestinal metaplasia for gastric cancer: Multicenter observation study in Japan.Dig Endosc. 2022 Mar;34(3):508-516. doi: 10.1111/den.14114. Epub 2021 Sep 16. Dig Endosc. 2022. PMID: 34415621
-
Assessment of Endoscopic Gastric Atrophy according to the Kimura-Takemoto Classification and Its Potential Application in Daily Practice.Clin Endosc. 2019 Jul;52(4):321-327. doi: 10.5946/ce.2019.072. Epub 2019 Jul 22. Clin Endosc. 2019. PMID: 31327182 Free PMC article. Review.
-
Endoscopic Kyoto classification of Helicobacter pylori infection and gastric cancer risk diagnosis.World J Gastroenterol. 2020 Feb 7;26(5):466-477. doi: 10.3748/wjg.v26.i5.466. World J Gastroenterol. 2020. PMID: 32089624 Free PMC article. Review.
References
-
- Chen PJ, Lin MC, Lai MJ, Lin JC, Lu HHS, Tseng VS. Accurate classification of diminutive colorectal polyps using computer‐aided analysis. Gastroenterology 2018; 154: 568–575. - PubMed
-
- Misawa M, Kudo SE, Mori Y et al. Artificial intelligence‐assisted polyp detection for colonoscopy: Initial experience. Gastroenterology 2018; 154: 2027–2029.e3. - PubMed
-
- Hirasawa T, Aoyama K, Tanimoto T et al. Application of artificial intelligence using a convolutional neural network for detecting gastric cancer in endoscopic images. Gastric Cancer 2018; 21: 653–660. - PubMed
-
- Shichijo S, Endo Y, Aoyama K et al. Application of convolutional neural networks for evaluating Helicobacter pylori infection status on the basis of endoscopic images. Scand J Gastroenterol 2019; 54: 158–163. - PubMed
LinkOut - more resources
Full Text Sources
