Amino acid deletions at positions 893 and 894 of cytotoxin-associated gene A protein affect Helicobacter pylori gastric epithelial cell interactions
- PMID: 39534413
- PMCID: PMC11551673
- DOI: 10.3748/wjg.v30.i41.4449
Amino acid deletions at positions 893 and 894 of cytotoxin-associated gene A protein affect Helicobacter pylori gastric epithelial cell interactions
Abstract
Background: Helicobacter pylori (H. pylori) persistently colonizes the human gastric mucosa in more than 50% of the global population, leading to various gastroduodenal diseases ranging from chronic gastritis to gastric carcinoma. Cytotoxin-associated gene A (CagA) protein, an important oncoprotein, has highly polymorphic Glu-Pro-Ile-Tyr-Ala segments at the carboxyl terminus, which play crucial roles in pathogenesis. Our previous study revealed a significant association between amino acid deletions at positions 893 and 894 and gastric cancer.
Aim: To investigate the impact of amino acid deletions at positions 893 and 894 on CagA function.
Methods: We selected a representative HZT strain from a gastric cancer patient with amino acid deletions at positions 893 and 894. The cagA gene was amplified and mutated into cagA-NT and cagA-NE (sequence characteristics of strains from nongastric cancer patients), cloned and inserted into pAdtrack-CMV, and then transfected into AGS cells. The expression of cagA and its mutants was examined using real-time polymerase chain reaction and Western blotting, cell elongation via cell counting, F-actin cytoskeleton visualization using fluorescence staining, and interleukin-8 (IL-8) secretion via enzyme-linked immunosorbent assay.
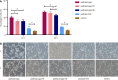
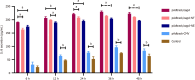
Results: The results revealed that pAdtrack/cagA induced a more pronounced hummingbird phenotype than pAdtrack/cagA-NT and pAdtrack/cagA-NE (40.88 ± 3.10 vs 32.50 ± 3.17, P < 0.001 and 40.88 ± 3.10 vs 32.17 ± 3.00, P < 0.001) at 12 hours after transfection. At 24 hours, pAdtrack/cagA-NE induced significantly fewer hummingbird phenotypes than pAdtrack/cagA and pAdtrack/cagA-NT (46.02 ± 2.12 vs 53.90 ± 2.10, P < 0.001 and 46.02 ± 2.12 vs 51.15 ± 3.74, P < 0.001). The total amount of F-actin caused by pAdtrack/cagA was significantly lower than that caused by pAdtrack/cagA-NT and pAdtrack/cagA-NE (27.54 ± 17.37 vs 41.51 ± 11.90, P < 0.001 and 27.54 ± 17.37 vs 41.39 ± 14.22, P < 0.001) at 12 hours after transfection. Additionally, pAdtrack/cagA induced higher IL-8 secretion than pAdtrack/cagA-NT and pAdtrack/cagA-NE at different times after transfection.
Conclusion: Amino acid deletions at positions 893 and 894 enhance CagA pathogenicity, which is crucial for revealing the pathogenic mechanism of CagA and identifying biomarkers of highly pathogenic H. pylori.
Keywords: Cytotoxin-associated gene A; Glu-Pro-Ile-Tyr-Ala; Helicobacter pylori; Hummingbird phenotype; Interleukin-8.
©The Author(s) 2024. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: The authors have no conflicts of interest and financial disclosures.
Figures




References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

