Trypsin in pancreatitis: The culprit, a mediator, or epiphenomenon?
- PMID: 39534420
- PMCID: PMC11551668
- DOI: 10.3748/wjg.v30.i41.4417
Trypsin in pancreatitis: The culprit, a mediator, or epiphenomenon?
Abstract
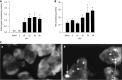
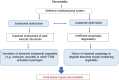
Pancreatitis is a common, life-threatening inflammatory disease of the exocrine pancreas. Its pathogenesis remains obscure, and no specific or effective treatment is available. Gallstones and alcohol excess are major etiologies of pancreatitis; in a small portion of patients the disease is hereditary. Pancreatitis is believed to be initiated by injured acinar cells (the main exocrine pancreas cell type), leading to parenchymal necrosis and local and systemic inflammation. The primary function of these cells is to produce, store, and secrete a variety of enzymes that break down all categories of nutrients. Most digestive enzymes, including all proteases, are secreted by acinar cells as inactive proforms (zymogens) and in physiological conditions are only activated when reaching the intestine. The generation of trypsin from inactive trypsinogen in the intestine plays a critical role in physiological activation of other zymogens. It was proposed that pancreatitis results from proteolytic autodigestion of the gland, mediated by premature/inappropriate trypsinogen activation within acinar cells. The intra-acinar trypsinogen activation is observed in experimental models of acute and chronic pancreatitis, and in human disease. On the basis of these observations, it has been considered the central pathogenic mechanism of pancreatitis - a concept with a century-old history. This review summarizes the data on trypsinogen activation in experimental and genetic rodent models of pancreatitis, particularly the more recent genetically engineered mouse models that mimic mutations associated with hereditary pancreatitis; analyzes the mechanisms mediating trypsinogen activation and protecting the pancreas against its' damaging effects; discusses the gaps in our knowledge, potential therapeutic approaches, and directions for future research. We conclude that trypsin is not the culprit in the disease pathogenesis but, at most, a mediator of some pancreatitis responses. Therefore, the search for effective therapies should focus on approaches to prevent or normalize other intra-acinar pathologic processes, such as defective autophagy leading to parenchymal cell death and unrelenting inflammation.
Keywords: Autophagy; Cathepsin; Cerulein; Cholecystokinin; Endolysosomal system; Hereditary pancreatitis; Pancreatic acinar cell.
©The Author(s) 2024. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Figures

References
-
- Pandol SJ, Saluja AK, Imrie CW, Banks PA. Acute pancreatitis: bench to the bedside. Gastroenterology. 2007;132:1127–1151. - PubMed
-
- Lee PJ, Papachristou GI. New insights into acute pancreatitis. Nat Rev Gastroenterol Hepatol. 2019;16:479–496. - PubMed
-
- Peery AF, Crockett SD, Murphy CC, Jensen ET, Kim HP, Egberg MD, Lund JL, Moon AM, Pate V, Barnes EL, Schlusser CL, Baron TH, Shaheen NJ, Sandler RS. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2021. Gastroenterology. 2022;162:621–644. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

