Breast ultrasound knobology and the knobology of twinkling for marker detection
- PMID: 39534581
- PMCID: PMC11557156
- DOI: 10.21037/tbcr-24-30
Breast ultrasound knobology and the knobology of twinkling for marker detection
Abstract
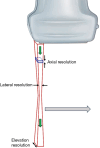
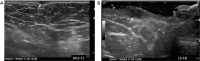
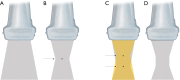
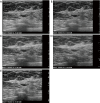
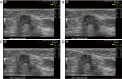
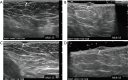
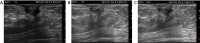
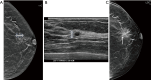
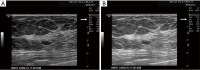
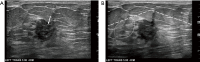
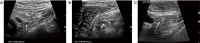
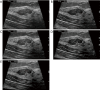
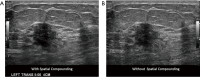
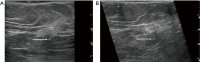
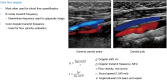
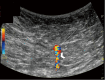
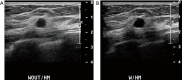
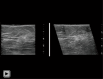
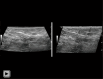
Breast ultrasound utilizes various scanning techniques to acquire optimal images for diagnostic evaluation. During interventional procedures, such as ultrasound-guided biopsies or preoperative localizations, knowledgeable and purposeful scanning adjustments are critical for successfully identifying the targeted mass or biopsy marker or clip. While most ultrasound scanning parameters are similar across different vendors, detailed descriptions specifically addressing the scanning parameters-often referred to as "knobology"- for breast ultrasound is at best limited in the literature. This review highlights ten key operator-controlled adjustments (including transducer selection, beam focusing, power, depth, gain and time gain compensation, harmonic imaging, spatial compounding, dynamic range, beam steering, and color Doppler) that significantly influence image quality in breast ultrasound. Each adjustment is accompanied by an "In practice" section providing examples and practical tips on implementation. The last topic discusses color Doppler which is generally used in breast ultrasound for evaluating the vascularity of a finding. Color Doppler, or more specifically, color Doppler twinkling, can be leveraged as a technique to detect certain breast biopsy markers that are challenging to detect by conventional B-mode ultrasound. While the cause of color Doppler twinkling is still under active investigation, twinkling is a clinically well-known, compelling ultrasound feature typically described with kidney stones. A step-by-step guide on how to use color Doppler twinkling to detect these markers is provided.
Keywords: Breast ultrasound; color Doppler twinkling; knobology; twinkling; ultrasound scanning.
2024 AME Publishing Company. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tbcr.amegroups.org/article/view/10.21037/tbcr-24-30/coif). C.U.L. serves as an unpaid editorial board member of Translational Breast Cancer Research from October 2023 to September 2025. C.U.L. and M.W.U. are co-PIs on a National Institute of Biomedical Imaging and Bioengineering (NIBIB) grant (R01 EB033008) to the Mayo Clinic and on an internal Mayo Clinic Discovery Translation Program award. Patents are pending for non-metallic ultrasound detectable markers as well as Doppler ultrasound twinkling technologies. G.K.H. was supported by NIBIB grant (R01 EB033008) to the Mayo Clinic and by an internal Mayo Clinic Discovery Translation Program award. The other authors have no other conflicts of interest to declare.
Figures












References
-
- WILD JJ , NEAL D. Use of high-frequency ultrasonic waves for detecting changes of texture in living tissues. Lancet 1951;1:655-7. - PubMed
-
- Lee CS, Bohm-Velez M. ACR Practice Parameter for the Performance of a Diagnostic Breast Ultrasound Examination. Am Coll Radiol 2021. Available online: https://www.acr.org/-/media/ACR/Files/Practice-Parameters/US-Breast.pdf
Publication types
LinkOut - more resources
Full Text Sources
