Decongestant Effect of "Coldamaris Akut", a Carrageenan- and Sorbitol-Containing Nasal Spray in Seasonal Allergic Rhinitis
- PMID: 39534593
- PMCID: PMC11556324
- DOI: 10.2147/IJGM.S476707
Decongestant Effect of "Coldamaris Akut", a Carrageenan- and Sorbitol-Containing Nasal Spray in Seasonal Allergic Rhinitis
Abstract
Purpose: This study aimed to develop a hyperosmolar, barrier-forming nasal spray based on carrageenan and sorbitol, and to demonstrate its decongestant effect in the context of allergic rhinitis (AR).
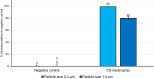
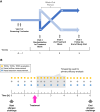
Methods: The efficacy of the nasal spray components was tested in vitro by barrier function, virus replication inhibition, and water absorption assays. The decongestant effectiveness was assessed in a randomized, controlled, crossover environmental chamber trial, where participants with a history of seasonal grass pollen AR were exposed to grass pollen allergens under controlled conditions. Forty-one adults were randomized to receive either carrageenan- and sorbitol-containing nasal spray (CS) or saline solution (SS). After 1 week, participants repeated the exposure with the treatment they had not received before. The primary efficacy endpoint was the mean change in nasal congestion symptom score (NCSS). Secondary efficacy endpoints were nasal airflow, nasal secretion, total nasal symptom score (TNSS), total ocular symptom score (TOSS) and total respiratory symptom score (TRSS).
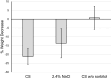
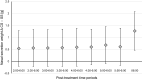
Results: Preclinical assays demonstrated barrier-building, virus-blocking, and water-withdrawing properties of the CS components. In the clinical study, there was no significant difference in mean NCSS change from pre- to post-treatment between CS and SS. However, nasal airflow increased over time after treatment with CS, while it declined after SS, leading to a growing difference in airflow between CS and SS (p = 0.04 at 6:00 h). Mean nasal secretion over 2-6 h was reduced by ~25% after CS (p = 0.003) compared to pre-treatment, while it was reduced by only ~16% after SS (p = 0.137). No significant differences in TNSS, TOSS and TRSS were observed between CS and SS.
Conclusion: CS improves nasal airflow and reduces nasal secretion in adults with AR. We propose CS as a safe and effective adjuvant to baseline pharmacological treatments.
Trial registration: NCT04532762.
Keywords: Allergic rhinitis; Carragelose; barrier; carrageenan; drug-free; nonpharmacological.
© 2024 Unger-Manhart et al.
Conflict of interest statement
NUM, MMK, HD, AR, PG, CK, CS, MKS and EPG are employees of Marinomed Biotech AG. CG is a former employee of Marinomed Biotech AG. MS received consulting fees from Marinomed Biotech AG. In addition, Dr Martina Morokutti-Kurz, Ms Christiane Koller, and Dr Eva Prieschl-Grassauer report a patent WO2017009351. The other authors have no competing interests in this work.
Figures



References
-
- Graf P. Long-term use of oxy- and xylometazoline nasal sprays induces rebound swelling, tolerance, and nasal hyperreactivity. Rhinology. 1996;34(1):9–13. - PubMed
-
- Akerlund A, Bende M. Sustained use of xylometazoline nose drops aggravates vasomotor rhinitis. Am J Rhinol. 1991;5(4):157–160.
Publication types
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

