Metabolic pathways fueling the suppressive activity of myeloid-derived suppressor cells
- PMID: 39534601
- PMCID: PMC11554506
- DOI: 10.3389/fimmu.2024.1461455
Metabolic pathways fueling the suppressive activity of myeloid-derived suppressor cells
Abstract
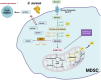
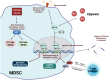
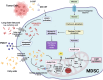
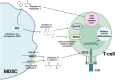
Myeloid-derived suppressor cells (MDSC) are considered an aberrant population of immature myeloid cells that have attracted considerable attention in recent years due to their potent immunosuppressive activity. These cells are typically absent or present in very low numbers in healthy individuals but become abundant under pathological conditions such as chronic infection, chronic inflammation and cancer. The immunosuppressive activity of MDSC helps to control excessive immune responses that might otherwise lead to tissue damage. This same immunosuppressive activity can be detrimental, particularly in cancer and chronic infection. In the cancer setting, tumors can secrete factors that promote the expansion and recruitment of MDSC, thereby creating a local environment that favors tumor progression by inhibiting the effective immune responses against cancer cells. This has made MDSC a target of interest in cancer therapy, with researchers exploring strategies to inhibit their function or reduce their numbers to improve the efficacy of cancer immunotherapies. In the context of chronic infections, MDSC can lead to persistent infections by suppressing protective immune responses thereby preventing the clearance of pathogens. Therefore, targeting MDSC may provide a novel approach to improve pathogen clearance during chronic infections. Ongoing research on MDSC aims to elucidate the exact processes behind their expansion, recruitment, activation and suppressive mechanisms. In this context, it is becoming increasingly clear that the metabolism of MDSC is closely linked to their immunosuppressive function. For example, MDSC exhibit high rates of glycolysis, which not only provides energy but also generates metabolites that facilitate their immunosuppressive activity. In addition, fatty acid metabolic pathways, such as fatty acid oxidation (FAO), have been implicated in the regulation of MDSC suppressive activity. Furthermore, amino acid metabolism, particularly arginine metabolism mediated by enzymes such as arginase-1, plays a critical role in MDSC-mediated immunosuppression. In this review, we discuss the metabolic signature of MDSC and highlight the therapeutic implications of targeting MDSC metabolism as a novel approach to modulate their immunosuppressive functions.
Keywords: immunosuppression; infection; metabolic reprogramming; metabolism; myeloid-derived suppressor cells; tumor.
Copyright © 2024 Goldmann and Medina.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
A Complex Metabolic Network Confers Immunosuppressive Functions to Myeloid-Derived Suppressor Cells (MDSCs) within the Tumour Microenvironment.Cells. 2021 Oct 9;10(10):2700. doi: 10.3390/cells10102700. Cells. 2021. PMID: 34685679 Free PMC article. Review.
-
Metabolic regulation of myeloid-derived suppressor cells in tumor immune microenvironment: targets and therapeutic strategies.Theranostics. 2025 Jan 13;15(6):2159-2184. doi: 10.7150/thno.105276. eCollection 2025. Theranostics. 2025. PMID: 39990210 Free PMC article. Review.
-
Myeloid-Derived Suppressor Cells: Immune-Suppressive Cells That Impair Antitumor Immunity and Are Sculpted by Their Environment.J Immunol. 2018 Jan 15;200(2):422-431. doi: 10.4049/jimmunol.1701019. J Immunol. 2018. PMID: 29311384 Free PMC article. Review.
-
Metabolic Regulation of Myeloid-Derived Suppressor Cell Function in Cancer.Cells. 2020 Apr 18;9(4):1011. doi: 10.3390/cells9041011. Cells. 2020. PMID: 32325683 Free PMC article. Review.
-
Lipid Metabolic Pathways Confer the Immunosuppressive Function of Myeloid-Derived Suppressor Cells in Tumor.Front Immunol. 2019 Jun 19;10:1399. doi: 10.3389/fimmu.2019.01399. eCollection 2019. Front Immunol. 2019. PMID: 31275326 Free PMC article. Review.
Cited by
-
Uncovering common transcriptional features shared by mature peripheral blood PMN-MDSCs and tumor-infiltrating neutrophils in humans.Oncoimmunology. 2025 Dec;14(1):2521396. doi: 10.1080/2162402X.2025.2521396. Epub 2025 Jul 1. Oncoimmunology. 2025. PMID: 40590753 Free PMC article.
-
Plasticity of myeloid-derived suppressor cells in cancer and cancer therapy.Oncol Res. 2025 Jun 26;33(7):1581-1592. doi: 10.32604/or.2025.060063. eCollection 2025. Oncol Res. 2025. PMID: 40612857 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

