Catechol-rich gelatin microspheres as restorative medical implants intended for inhibiting seroma formation and promoting wound healing
- PMID: 39534679
- PMCID: PMC11554634
- DOI: 10.1016/j.mtbio.2024.101313
Catechol-rich gelatin microspheres as restorative medical implants intended for inhibiting seroma formation and promoting wound healing
Abstract
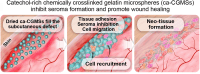
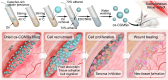
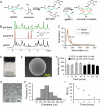
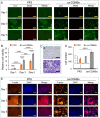
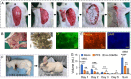
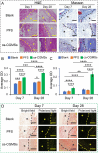
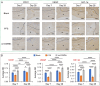
Seroma formation and poor wound healing are common complications of many surgeries that create anatomical dead space (i.e., mastectomy), often causing tissue infection and even necrosis. Although negative pressure drainage and tissue adhesives are investigated to alleviate fluid accumulation post-surgery, however, their therapeutic efficacy remains unsatisfactory in most cases. Herein, the catechol-rich chemically crosslinked gelatin microspheres (ca-CGMSs) have been developed as biodegradable reconstructive implants for preventing seroma formation and concurrently promoting subcutaneous wound healing. Compared with the most representative hydrogel adhesive, i.e. commercial porcine fibrin sealant (PFS), the loosely packed ca-CGMSs with diameters range from 50 to 350 μm, provide numerous cell-adhesive interfaces and interconnected macro-pores for enhanced cell adhesion, proliferation and migration. Subcutaneous embedding trials show the in situ swelling aggregation and wet tissue adhesion of ca-CGMSs as well as their capacity in recruiting autologous cells in rat mastectomy models. The trials in rabbit mastectomy models demonstrate that, compared with PFS gluing, the implanted dried ca-CGMSs not only significantly inhibit seroma formation, but also achieve enhanced wound healing by inducing the formation of vascularized neo-tissue. The ca-CGMSs show a great potential to be the next-generation of restorative materials for both preventing seroma formation and healing subcutaneous wounds.
Keywords: Catechol; Gelatin; Microsphere; Seroma; Tissue engineering; Wound healing.
© 2024 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
A mussel-inspired double-crosslinked tissue adhesive intended for internal medical use.Acta Biomater. 2016 Mar;33:51-63. doi: 10.1016/j.actbio.2016.02.003. Epub 2016 Feb 2. Acta Biomater. 2016. PMID: 26850148
-
A mussel-inspired double-crosslinked tissue adhesive on rat mastectomy model: seroma prevention and in vivo biocompatibility.J Surg Res. 2017 Jul;215:173-182. doi: 10.1016/j.jss.2017.03.020. Epub 2017 Mar 31. J Surg Res. 2017. PMID: 28688644
-
Multi-crosslinked hydrogels with strong wet adhesion, self-healing, antibacterial property, reactive oxygen species scavenging activity, and on-demand removability for seawater-immersed wound healing.Acta Biomater. 2023 Mar 15;159:95-110. doi: 10.1016/j.actbio.2023.01.045. Epub 2023 Feb 2. Acta Biomater. 2023. PMID: 36736644
-
Effects of Fibrin Sealant on Seroma Reduction for Patients with Breast Cancer Undergoing Axillary Dissection: Meta-Analysis of Randomized Controlled Trials.Ann Surg Oncol. 2020 Dec;27(13):5286-5295. doi: 10.1245/s10434-020-08747-5. Epub 2020 Jun 20. Ann Surg Oncol. 2020. PMID: 32564232
-
Bio-inspired adhesive catechol-conjugated chitosan for biomedical applications: A mini review.Acta Biomater. 2015 Nov;27:101-115. doi: 10.1016/j.actbio.2015.08.043. Epub 2015 Aug 28. Acta Biomater. 2015. PMID: 26318801 Review.
Cited by
-
Antibacterial and Cell-Adhesive Poly(2-ethyl-2-oxazoline) Hydrogels Developed for Wound Treatment: In Vitro Evaluation.Biomacromolecules. 2025 May 12;26(5):3139-3154. doi: 10.1021/acs.biomac.5c00181. Epub 2025 Apr 29. Biomacromolecules. 2025. PMID: 40298243 Free PMC article.
References
-
- Bertucci F., Ng C.K.Y., Patsouris A., Droin N., Piscuoglio S., Carbuccia N., Soria J.C., Dien A.T., Adnani Y., Kamal M., Garnier S., Meurice G., Jimenez M., Dogan S., Verret B., Chaffanet M., Bachelot T., Campone M., Lefeuvre C., Bonnefoi H., Dalenc F., Jacquet A., De Filippo M.R., Babbar N., Birnbaum D., Filleron T., Le Tourneau C., Andre F. Genomic characterization of metastatic breast cancers. Nature. 2019;569(7757):560. doi: 10.1038/s41586-019-1056-z. - DOI - PubMed
-
- De Rooij L., van Kuijk S.M.J., van Haaren E.R.M., Janssen A., Vissers Y.L.J., Beets G.L., van Bastelaar J. Negative pressure wound therapy does not decrease postoperative wound complications in patients undergoing mastectomy and flap fixation. Sci. Rep. 2021;11(1):9620. doi: 10.1038/s41598-021-89036-3. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

