Development and preliminary assessment of the iFIND TBR: all-in- one molecular diagnostic assay for rapid detection of Mycobacterium tuberculosis and rifampicin resistance
- PMID: 39534701
- PMCID: PMC11554655
- DOI: 10.3389/fcimb.2024.1439099
Development and preliminary assessment of the iFIND TBR: all-in- one molecular diagnostic assay for rapid detection of Mycobacterium tuberculosis and rifampicin resistance
Abstract
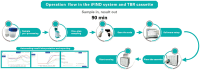
Introduction: Early and accurate diagnosis of tuberculosis (TB) is crucial for initiating timely treatment and preventing new infections. In this study, we introduced the iFIND TBR assay, an automated all-in-one tuberculosis detection approach that simultaneously detect Mycobacterium tuberculosis (MTB) and rifampicin (RIF) resistance.
Methods: The limits of detection (LOD), sensitivity, specificity, and RIF-R rpoB mutation detection of the iFIND TBR were tested on Mycobacterium tuberculosis DNA or sputum samples spiked with known numbers of M.tuberculosis H37Rv. Frozen clinical samples from patients suspected of having TB were also tested.
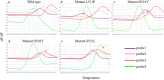
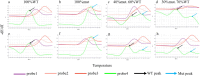
Results: The LOD of the iFIND TBR for MTB detection were 13.34 CFU/ml (95% CI, 11.71-16.47), and for RIF resistance was 109.79CFU/mL (95% CI, 95-138.19). The iFIND TBR assay accurately distinguish MTB strains from non-tuberculous mycobacteria (NTM) without any cross reactivity. Testing on 157 clinical sputum samples, compared with the bacteriologically TB standard, the overall sensitivity and specificity of the iFIND TBR was 100% (95%CI, 94.64, 100) and 85.29% (95% CI, 74.61, 92.72), respectively. When assessing RIF susceptibility, the iFIND TBR achieved a sensitivity of 98.15% (95% CI, 90.11-99.95) and a specificity of 85.71% (95% CI, 67.33-95.97), compared with phenotypic drug susceptibility testing. Discordant RIF susceptibility results were more frequently observed in samples exhibiting heteroresistance.
Discussion: These findings demonstrate that iFIND TBR assay performs well in detecting TB and RIF resistance, and shows promise as a point-of-care tool in resource-limited areas.
Keywords: Mycobacterium tuberculosis; rapid detection; rifampicin resistance; the iFIND TBR; tuberculosis.
Copyright © 2024 Ou, Song, Xing, Zhao, Pei, Teng, Zhang, Sun, Liu, Xia, Zhou, Zheng, Song, Zhang, Wang, Anthony and Zhao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Analytical and clinical validation of a novel MeltPlus TB-NTM/RIF platform for simultaneous detection of Mycobacterium tuberculosis complex, Non-Tuberculous Mycobacteria and rifampicin resistance.Front Cell Infect Microbiol. 2025 Feb 10;15:1534268. doi: 10.3389/fcimb.2025.1534268. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 39996132 Free PMC article.
-
The New Xpert MTB/RIF Ultra: Improving Detection of Mycobacterium tuberculosis and Resistance to Rifampin in an Assay Suitable for Point-of-Care Testing.mBio. 2017 Aug 29;8(4):e00812-17. doi: 10.1128/mBio.00812-17. mBio. 2017. PMID: 28851844 Free PMC article.
-
Comparison of targeted next-generation sequencing and the Xpert MTB/RIF assay for detection of Mycobacterium tuberculosis in clinical isolates and sputum specimens.Microbiol Spectr. 2024 May 2;12(5):e0409823. doi: 10.1128/spectrum.04098-23. Epub 2024 Apr 11. Microbiol Spectr. 2024. PMID: 38602399 Free PMC article.
-
Xpert MTB/RIF and Xpert MTB/RIF Ultra assays for active tuberculosis and rifampicin resistance in children.Cochrane Database Syst Rev. 2020 Aug 27;8(8):CD013359. doi: 10.1002/14651858.CD013359.pub2. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2022 Sep 6;9:CD013359. doi: 10.1002/14651858.CD013359.pub3. PMID: 32853411 Free PMC article. Updated.
-
Xpert® MTB/RIF: Usefulness for the diagnosis of tuberculosis and resistance to rifampicin.Med Clin (Barc). 2017 Nov 9;149(9):399-405. doi: 10.1016/j.medcli.2017.06.007. Epub 2017 Jul 22. Med Clin (Barc). 2017. PMID: 28739268 Review. English, Spanish.
Cited by
-
Analytical and clinical validation of a novel MeltPlus TB-NTM/RIF platform for simultaneous detection of Mycobacterium tuberculosis complex, Non-Tuberculous Mycobacteria and rifampicin resistance.Front Cell Infect Microbiol. 2025 Feb 10;15:1534268. doi: 10.3389/fcimb.2025.1534268. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 39996132 Free PMC article.
References
-
- WHO . (2014). “WHO guidelines approved by the guidelines review committee,” in Xpert mtb/rif implementation manual: technical and operational ‘how-to’; practical considerations (Geneva: World Health Organization; ). - PubMed
-
- Chakravorty S., Simmons A. M., Rowneki M., Parmar H., Cao Y., Ryan J., et al. . (2017). The new Xpert MTB/RIF ultra: improving detection of mycobacterium tuberculosis and resistance to rifampin in an assay suitable for point-of-care testing. mBio 8(4). doi: 10.1128/mBio.00812-17 - DOI - PMC - PubMed
-
- Deng Y., Ma Z., Su B., Bai G., Pan J., Wang Q., et al. . (2023). Accuracy of the InnowaveDX MTB/RIF test for detection of Mycobacterium tuberculosis and rifampicin resistance: a prospective multicentre study. Emerg. Microbes Infect. 12 (1), 2151382. doi: 10.1080/22221751.2022.2151382 - DOI - PMC - PubMed
-
- He W., Tan Y., Liu C., Wang Y., He P., Song Z., et al. . (2022). Drug-resistant characteristics, genetic diversity, and transmission dynamics of rifampicin-resistant mycobacterium tuberculosis in Hunan, China, revealed by whole-genome sequencing. Microbiol. Spectr. 10 (1), e0154321. doi: 10.1128/spectrum.01543-21 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

