NFE2L2 and ferroptosis resistance in cancer therapy
- PMID: 39534872
- PMCID: PMC11555182
- DOI: 10.20517/cdr.2024.123
NFE2L2 and ferroptosis resistance in cancer therapy
Abstract
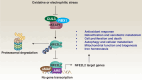
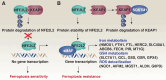
NFE2-like basic leucine zipper transcription factor 2 (NFE2L2, also known as NRF2), is a key transcription factor in the cellular defense against oxidative stress, playing a crucial role in cancer cell survival and resistance to therapies. This review outlines the current knowledge on the link between NFE2L2 and ferroptosis - a form of regulated cell death characterized by iron-dependent lipid peroxidation - within cancer cells. While NFE2L2 activation can protect normal cells from oxidative damage, its overexpression in cancer cells contributes to drug resistance by upregulating antioxidant defenses and inhibiting ferroptosis. We delve into the molecular pathways of ferroptosis, highlighting the involvement of NFE2L2 and its target genes, such as NQO1, HMOX1, FTH1, FTL, HERC2, SLC40A1, ABCB6, FECH, PIR, MT1G, SLC7A11, GCL, GSS, GSR, GPX4, AIFM2, MGST1, ALDH1A1, ALDH3A1, and G6PD, in ferroptosis resistance. Understanding the delicate balance between NFE2L2's protective and deleterious roles could pave the way for novel therapeutic strategies targeting NFE2L2 to enhance the efficacy of ferroptosis inducers in cancer therapy.
Keywords: Cancer therapy; NFE2L2; drug resistance; ferroptosis; oxidative stress.
© The Author(s) 2024.
Conflict of interest statement
Both authors declared that there are no conflicts of interest.
Figures

Similar articles
-
Ferroptosis model system by the re-expression of BACH1.J Biochem. 2023 Jul 31;174(3):239-252. doi: 10.1093/jb/mvad036. J Biochem. 2023. PMID: 37094356
-
JAK/STAT signaling as a key regulator of ferroptosis: mechanisms and therapeutic potentials in cancer and diseases.Cancer Cell Int. 2025 Mar 7;25(1):83. doi: 10.1186/s12935-025-03681-6. Cancer Cell Int. 2025. PMID: 40055704 Free PMC article. Review.
-
Ferroptosis regulation by Cap'n'collar family transcription factors.J Biol Chem. 2024 Aug;300(8):107583. doi: 10.1016/j.jbc.2024.107583. Epub 2024 Jul 16. J Biol Chem. 2024. PMID: 39025451 Free PMC article. Review.
-
MGST1 is a redox-sensitive repressor of ferroptosis in pancreatic cancer cells.Cell Chem Biol. 2021 Jun 17;28(6):765-775.e5. doi: 10.1016/j.chembiol.2021.01.006. Epub 2021 Feb 3. Cell Chem Biol. 2021. PMID: 33539732
-
Mechanism of ferroptosis resistance in cancer cells.Cancer Drug Resist. 2024 Nov 20;7:47. doi: 10.20517/cdr.2024.127. eCollection 2024. Cancer Drug Resist. 2024. PMID: 39624080 Free PMC article. Review.
Cited by
-
Ferroptosis and Nrf2 Signaling in Head and Neck Cancer: Resistance Mechanisms and Therapeutic Prospects.Antioxidants (Basel). 2025 Aug 13;14(8):993. doi: 10.3390/antiox14080993. Antioxidants (Basel). 2025. PMID: 40867889 Free PMC article. Review.
-
Chemical Inhibition of NRF2 Transcriptional Activity Influences Colon Function and Oestrogen Receptor Expression in Mice at Different Ages.Int J Mol Sci. 2024 Dec 20;25(24):13647. doi: 10.3390/ijms252413647. Int J Mol Sci. 2024. PMID: 39769410 Free PMC article.
-
Non‑coding RNA‑mediated epigenetic modification of ferroptosis in non‑small cell lung cancer (Review).Int J Oncol. 2025 Jan;66(1):8. doi: 10.3892/ijo.2024.5714. Epub 2024 Dec 13. Int J Oncol. 2025. PMID: 39670309 Free PMC article. Review.
-
Macrophages and macrophage extracellular vesicles confer cancer ferroptosis resistance via PRDX6-mediated mitophagy inhibition.Redox Biol. 2025 Aug 16;86:103826. doi: 10.1016/j.redox.2025.103826. Online ahead of print. Redox Biol. 2025. PMID: 40825268 Free PMC article.
-
PIP5K1A Suppresses Ferroptosis and Induces Sorafenib Resistance by Stabilizing NRF2 in Hepatocellular Carcinoma.Adv Sci (Weinh). 2025 Aug;12(30):e04372. doi: 10.1002/advs.202504372. Epub 2025 May 23. Adv Sci (Weinh). 2025. PMID: 40405713 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
