Adenoviral delivery of the CIITA transgene induces T-cell-mediated killing in glioblastoma organoids
- PMID: 39535369
- PMCID: PMC11887676
- DOI: 10.1002/1878-0261.13750
Adenoviral delivery of the CIITA transgene induces T-cell-mediated killing in glioblastoma organoids
Abstract
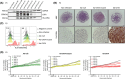
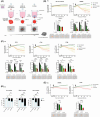
The immunosuppressive nature of the tumor microenvironment poses a significant challenge to effective immunotherapies against glioblastoma (GB). Boosting the immune response is critical for successful therapy. Here, we adopted a cancer gene therapy approach to induce T-cell-mediated killing of the tumor through increased activation of the immune system. Patient-based three-dimensional (3D) GB models were infected with a replication-deficient adenovirus (AdV) armed with the class II major histocompatibility complex (MHC-II) transactivator (CIITA) gene (Ad-CIITA). Successful induction of surface MHC-II was achieved in infected GB cell lines and primary human GB organoids. Infection with an AdV carrying a mutant form of CIITA with a single amino acid substitution resulted in cytoplasmic accumulation of CIITA without subsequent MHC-II expression. Co-culture of infected tumor cells with either peripheral blood mononuclear cells (PBMCs) or isolated T-cells led to dramatic breakdown of GB organoids. Intriguingly, both wild-type and mutant Ad-CIITA, but not unarmed AdV, triggered immune-mediated tumor cell death in the co-culture system, suggesting an at least partially MHC-II-independent process. We further show that the observed cancer cell killing requires the presence of either CD8+ or CD4+ T-cells and direct contact between GB and immune cells. We did not, however, detect evidence of activation of canonical T-cell-mediated cell death pathways. Although the precise mechanism remains to be determined, these findings highlight the potential of AdV-mediated CIITA delivery to enhance T-cell-mediated immunity against GB.
Keywords: MHC‐II; T‐cells; adenovirus; gene therapy; glioblastoma; tumor organoids.
© 2024 The Author(s). Molecular Oncology published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Non-Immune-Mediated, p27-Associated, Growth Inhibition of Glioblastoma by Class-II-Transactivator (CIITA).Cells. 2024 Nov 14;13(22):1883. doi: 10.3390/cells13221883. Cells. 2024. PMID: 39594630 Free PMC article.
-
Fowlpoxvirus recombinants coding for the CIITA gene increase the expression of endogenous MHC-II and Fowlpox Gag/Pro and Env SIV transgenes.PLoS One. 2018 Jan 31;13(1):e0190869. doi: 10.1371/journal.pone.0190869. eCollection 2018. PLoS One. 2018. PMID: 29385169 Free PMC article.
-
Introduction of the CIITA gene into tumor cells produces exosomes with enhanced anti-tumor effects.Exp Mol Med. 2011 May 31;43(5):281-90. doi: 10.3858/emm.2011.43.5.029. Exp Mol Med. 2011. PMID: 21464590 Free PMC article.
-
CIITA-Driven MHC Class II Expressing Tumor Cells as Antigen Presenting Cell Performers: Toward the Construction of an Optimal Anti-tumor Vaccine.Front Immunol. 2019 Jul 30;10:1806. doi: 10.3389/fimmu.2019.01806. eCollection 2019. Front Immunol. 2019. PMID: 31417570 Free PMC article. Review.
-
Expression of MHC II genes.Curr Top Microbiol Immunol. 2005;290:147-70. doi: 10.1007/3-540-26363-2_7. Curr Top Microbiol Immunol. 2005. PMID: 16480042 Review.
Cited by
-
Glioblastoma-instructed microglia transition to heterogeneous phenotypic states with phagocytic and dendritic cell-like features in patient tumors and patient-derived orthotopic xenografts.Genome Med. 2024 Apr 2;16(1):51. doi: 10.1186/s13073-024-01321-8. Genome Med. 2024. PMID: 38566128 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

