Efficacy and safety of tozorakimab in moderate-to-severe atopic dermatitis: A Phase 2a randomized controlled trial (FRONTIER-2)
- PMID: 39535462
- PMCID: PMC12105459
- DOI: 10.1111/jdv.20388
Efficacy and safety of tozorakimab in moderate-to-severe atopic dermatitis: A Phase 2a randomized controlled trial (FRONTIER-2)
Abstract
Background: Atopic dermatitis (AD) is a chronic, inflammatory skin disease characterized by intense pruritus and eczematous lesions. Tozorakimab is a high-affinity human monoclonal antibody that neutralizes interleukin-33, a broad-acting alarmin cytokine that is over-expressed in keratinocytes of patients with AD.
Objectives: This Phase 2a study (FRONTIER-2; NCT04212169) evaluated the safety and efficacy of tozorakimab in adults with moderate-to-severe AD.
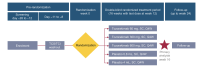
Methods: FRONTIER-2 was a randomized, placebo-controlled, parallel-group, double-blind study conducted from 9 December 2019 to 20 September 2022 at 32 centres across six countries. Patients were randomized 3:1:1:3 to receive placebo, tozorakimab 60 mg, tozorakimab 300 mg or tozorakimab 600 mg by subcutaneous injection once every 4 weeks for four doses. The primary endpoint was percentage change from baseline to Week 16 in the Eczema Area and Severity Index (EASI) score in patients treated with tozorakimab versus placebo. Secondary outcomes included EASI-75 responders (patients achieving ≥75% reduction from baseline in EASI score), Investigator's Global Assessment (IGA) responders (patients achieving an IGA score of 0 or 1), pharmacokinetics, immunogenicity and safety.
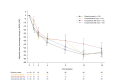
Results: Overall, 148 patients were randomized. There was no statistically significant difference in the primary endpoint (60 mg difference of 1.3 [90% confidence interval (CI): -13.7, 16.2], p = 0.888; 300 mg: difference of 5.9 [90% CI: -10.4, 22.1], p = 0.549; 600 mg: difference of - 1.7 [90% CI: -13.4, 10.0], p = 0.807). The proportion of EASI-75 and IGA 0/1 responders at Week 16 was numerically higher in the tozorakimab 600 mg group than in the placebo group (EASI-75: 18.2% vs. 7.1%, p = 0.094; IGA 0/1: 9.1% vs. 1.8%, p = 0.113). Serum pharmacokinetics were dose-dependent, immunogenicity incidence was low and tozorakimab was well tolerated.
Conclusions: FRONTIER-2 did not show a statistically significant difference in the primary endpoint for tozorakimab compared with placebo. However, numerical increases in responder rates were observed.
© 2024 AstraZeneca and The Author(s). Journal of the European Academy of Dermatology and Venereology published by John Wiley & Sons Ltd on behalf of European Academy of Dermatology and Venereology.
Conflict of interest statement
J.I.S. has received grant funding from Galderma, Incyte and Pfizer, for which his institution has been renumerated; received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events and participated on a Data Safety Monitoring Board or Advisory Board for AbbVie, Alamar, Aldena, Amgen, AOBiome, Apollo, Arcutis, Arena, Asana, Aslan, Attovia, BiomX, Biosion, Bodewell, Boehringer‐Ingelheim, Bristol‐Meyers Squibb, Cara, Castle Biosciences, Celgene, Connect Biopharma, CorEvitas, Dermavant, Eli Lilly, FIDE, Galderma, GlaxoSmithKline, Incyte, Inmagene, Invea, Kiniksa, Leo Pharma, Merck, MyOr Diagnostics, Nektar, Novartis, Optum, Pfizer, RAPT, Recludix, Regeneron, Sandoz, Sanofi‐Genzyme, Shaperon, TARGET‐RWE, Teva, Union and UpToDate; and has declared other financial or non‐financial interests from AbbVie, Eli Lilly, Leo Pharma, Pfizer, Regeneron and Sanofi‐Genzyme. M.N.M., H.C.P., F.R., A.L., R.S., R.M., A.K., E.J., M.G.B, M.W.S. and C.K. are employees of AstraZeneca and may hold stock or stock options. R.C. and M.G. are former employees of AstraZeneca and may hold stock or stock options.
Figures
Similar articles
-
Long-term management of moderate-to-severe atopic dermatitis with lebrikizumab and concomitant topical corticosteroids: a 68-week randomized double-blind placebo-controlled phase III trial in Japan (ADhere-J).Br J Dermatol. 2025 Mar 18;192(4):597-610. doi: 10.1093/bjd/ljae394. Br J Dermatol. 2025. PMID: 39442013 Clinical Trial.
-
Efficacy and Safety of Upadacitinib versus Dupilumab Treatment for Moderate-to-Severe Atopic Dermatitis in Four Body Regions: Analysis from the Heads Up Study.Dermatology. 2025;241(1):10-18. doi: 10.1159/000542275. Epub 2024 Oct 30. Dermatology. 2025. PMID: 39476813 Free PMC article. Clinical Trial.
-
Benralizumab does not elicit therapeutic effect in patients with chronic spontaneous urticaria: results from the phase IIb multinational randomized double-blind placebo-controlled ARROYO trial.Br J Dermatol. 2024 Jul 16;191(2):187-199. doi: 10.1093/bjd/ljae067. Br J Dermatol. 2024. PMID: 38367194 Clinical Trial.
-
Systemic treatments for eczema: a network meta-analysis.Cochrane Database Syst Rev. 2020 Sep 14;9(9):CD013206. doi: 10.1002/14651858.CD013206.pub2. Cochrane Database Syst Rev. 2020. PMID: 32927498 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
References
-
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, Boyle J, Fonacier L, Gelfand JM, et al. Atopic dermatitis in America study: a cross‐sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139:583–590. - PubMed
-
- Davis DMR, Drucker AM, Alikhan A, Bercovitch L, Cohen DE, Darr JM, et al. Guidelines of care for the management of atopic dermatitis in adults with phototherapy and systemic therapies. J Am Acad Dermatol. 2024;90:e43–e56. - PubMed
-
- Simpson EL, Bieber T, Guttman‐Yassky E, Beck LA, Blauvelt A, Cork MJ, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335–2348. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

