Going Beyond Woodward and Hoffmann's Electrocyclizations and Cycloadditions: Sigmatropic Rearrangements
- PMID: 39535477
- PMCID: PMC11665502
- DOI: 10.1002/tcr.202400050
Going Beyond Woodward and Hoffmann's Electrocyclizations and Cycloadditions: Sigmatropic Rearrangements
Abstract
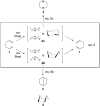
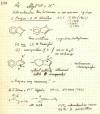
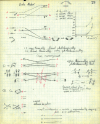
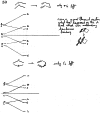
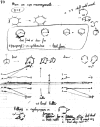
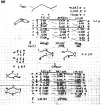
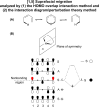
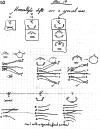
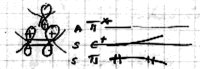
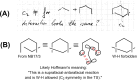
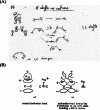
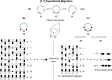
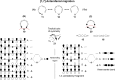
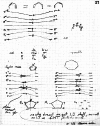
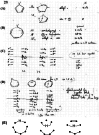
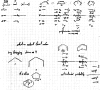
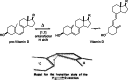
On June 1, 1965, R. B. Woodward and Roald Hoffmann published their third communication in the Journal of the American Chemical Society in which they applied orbital symmetry control to explain the mechanism of a wide variety of valence isomerizations that they termed "sigmatropic reactions." This publication reveals the research trajectory taken by Hoffmann from which this portion of the no-mechanism problem was solved. Hoffmann used five different quantum chemical tools, all based on either extended Hückel theoretical calculations or frontier molecular orbital theory, in his research. Hoffmann's laboratory notebooks and his three draft manuscripts along with Woodward's four subsequent drafts have survived the past 59 years and provide an excellent window into the thinking and manuscript-writing processes used by these Nobel laureates in February-April 1965.
Keywords: Conservation of Orbital Symmetry; Cope reaction; Frontier Molecular Orbital Theory; Woodward-Hoffmann rules; sigmatropic reactions.
© 2024 The Author(s). The Chemical Record published by The Chemical Society of Japan and Wiley-VCH GmbH.
Figures






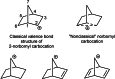

















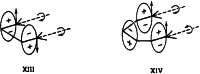












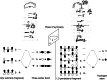




















References
-
- R. Hoffmann, email to J. I. Seeman, Ithaca, NY, June 21, 2019.
-
- R. Hoffmann, video interview with J. I. Seeman, Ithaca, NY, February 25, 2022.
-
- Woodward R. B., Hoffmann R., Angew. Chem. Int. Ed. 1969, 8, 781–853, 10.1002/anie.196907811. - DOI
-
- Woodward R. B., Hoffmann R., The Conservation of Orbital Symmetry, Verlag Chemie, Weinheim, 1970.
-
- Anonymous, Red herring, Wikipedia, 2024. https://en.wikipedia.org/wiki/Red_herring, accessed on August 5, 2024.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

