Large-scale deep proteomic analysis in Alzheimer's disease brain regions across race and ethnicity
- PMID: 39535480
- PMCID: PMC11667503
- DOI: 10.1002/alz.14360
Large-scale deep proteomic analysis in Alzheimer's disease brain regions across race and ethnicity
Abstract
Introduction: Alzheimer's disease (AD) is the most prevalent neurodegenerative disease, yet our comprehension predominantly relies on studies within non-Hispanic White (NHW) populations. Here we provide an extensive survey of the proteomic landscape of AD across diverse racial/ethnic groups.
Methods: Two cortical regions, from multiple centers, were harmonized by uniform neuropathological diagnosis. Among 998 unique donors, 273 donors self-identified as African American, 229 as Latino American, and 434 as NHW.
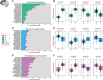
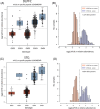
Results: While amyloid precursor protein and the microtubule-associated protein tau demonstrated higher abundance in AD brains, no significant race-related differences were observed. Further proteome-wide and focused analyses (specific amyloid beta [Aβ] species and the tau domains) supported the absence of racial differences in these AD pathologies within the brain proteome.
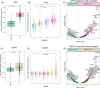
Discussion: Our findings indicate that the racial differences in AD risk and clinical presentation are not underpinned by dramatically divergent patterns in the brain proteome, suggesting that other determinants account for these clinical disparities.
Highlights: We present a large-scale proteome (∼10,000 proteins) of DLPFC (998) and STG (244) across AD cases. About 50% of samples were from racially and ethnically diverse brain donors. Key AD proteins (amyloid and tau) correlated with CERAD and Braak stages. No significant race-related differences in amyloid and tau protein levels were observed in AD brains. AD-associated protein changes showed a strong correlation between the brain proteomes of African American and White individuals. This dataset advances understanding of ethnoracial-specific AD pathways and potential therapies.
Keywords: Alzheimer's disease; data descriptor; diversity; precision medicine; proteome; proteomics.
© 2024 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
The authors declare no conflicts of interest. Author disclosures are available in the supporting information.
Figures




Update of
-
Large-scale Deep Proteomic Analysis in Alzheimer's Disease Brain Regions Across Race and Ethnicity.bioRxiv [Preprint]. 2024 Apr 26:2024.04.22.590547. doi: 10.1101/2024.04.22.590547. bioRxiv. 2024. Update in: Alzheimers Dement. 2024 Dec;20(12):8878-8897. doi: 10.1002/alz.14360. PMID: 38712030 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
- P30 AG072975/AG/NIA NIH HHS/United States
- IK4 BX005219/BX/BLRD VA/United States
- R01 AG053960/AG/NIA NIH HHS/United States
- R01 AG057911/AG/NIA NIH HHS/United States
- U01 AG061357/AG/NIA NIH HHS/United States
- P30 AG010161/AG/NIA NIH HHS/United States
- R01 NS080820/NS/NINDS NIH HHS/United States
- U19 AG062418/AG/NIA NIH HHS/United States
- R01 AG018023/AG/NIA NIH HHS/United States
- U01 AG046152/AG/NIA NIH HHS/United States
- U54 AG065187/AG/NIA NIH HHS/United States
- R01 AG061800/AG/NIA NIH HHS/United States
- RF1 AG062181/AG/NIA NIH HHS/United States
- P50 AG016574/AG/NIA NIH HHS/United States
- P30 AG066511/AG/NIA NIH HHS/United States
- U01 AG061356/AG/NIA NIH HHS/United States
- R01 AG072120/AG/NIA NIH HHS/United States
- U19 AG074879/AG/NIA NIH HHS/United States
- I01 BX003853/BX/BLRD VA/United States
- U24 AG061340/AG/NIA NIH HHS/United States
- R01 AG017917/AG/NIA NIH HHS/United States
- P30 AG072980/AG/NIA NIH HHS/United States
- R01 AG061796/AG/NIA NIH HHS/United States
- R01 AG032990/AG/NIA NIH HHS/United States
- P01 AG066597/AG/NIA NIH HHS/United States
- U01-AG061357/AG/NIA NIH HHS/United States
- RC2 AG036547/AG/NIA NIH HHS/United States
- R01 AG022018/AG/NIA NIH HHS/United States
- U01 AG046139/AG/NIA NIH HHS/United States
- P01 AG003949/AG/NIA NIH HHS/United States
- U24 NS072026/NS/NINDS NIH HHS/United States
- U01 AG046161/AG/NIA NIH HHS/United States
- P30 AG019610/AG/NIA NIH HHS/United States
- P50 AG025711/AG/NIA NIH HHS/United States
- R01 AG075827/AG/NIA NIH HHS/United States
- P30 AG072972/AG/NIA NIH HHS/United States
- P01 AG017216/AG/NIA NIH HHS/United States
- R01 AG079170/AG/NIA NIH HHS/United States
- U01 AG006786/AG/NIA NIH HHS/United States
- P30 AG072979/AG/NIA NIH HHS/United States
- R01 AG015819/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

