Real-World Comparative Effectiveness and Safety of Filgotinib and Upadacitinib for Ulcerative Colitis: A Multicentre Cohort Study
- PMID: 39535492
- PMCID: PMC11652322
- DOI: 10.1002/ueg2.12704
Real-World Comparative Effectiveness and Safety of Filgotinib and Upadacitinib for Ulcerative Colitis: A Multicentre Cohort Study
Abstract
Background: Janus kinase (JAK) inhibitors, filgotinib (FIL) and upadacitinib (UPA) have emerged as promising treatments for ulcerative colitis (UC). However, a comparative analysis of these JAK inhibitors, particularly in patients previously treated with tofacitinib (TOF), has not been performed.
Aims: To compare the efficacy and safety of FIL and UPA in patients with UC, including those previously exposed to TOF.
Methods: A multicentre retrospective cohort study was conducted to compare the effectiveness and safety of FIL and UPA in patients with UC whose treatment was initiated between March 2022 and December 2023. The co-primary outcomes were clinical response and remission at week 8. The secondary outcomes included treatment persistence and adverse events (AEs). Modified Poisson and Cox regression models with multivariable analysis to adjust for confounders and propensity score matching were conducted. Subgroup analyses stratified by previous exposure to TOF and biologics were also conducted.
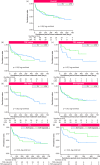
Results: In total, 168 patients (98 treated with FIL and 70 treated with UPA) were enrolled in this study, with a median follow-up period of 181 days. The clinical response/remission rates at week 8 were 55.1/46.9% for FIL and 71.4/65.7% for UPA, respectively. UPA was associated with significantly higher rates of clinical response (adjusted risk ratio [RR] 1.40 [95% confidence interval [CI], 1.09 to 1.80]) and clinical remission (adjusted RR 1.54 [95% CI, 1.16 to 2.05]) compared with FIL. This result was consistent across subgroup analyses based on previous exposure to TOF or biologics, except for bio-naive patients. There was no significant difference in the treatment persistence. AEs were more frequent with UPA (45.7%) than with FIL (24.5%) (p = 0.0049). Propensity score matching confirmed the superior overall effectiveness of UPA.
Conclusions: UPA demonstrated better short-term effectiveness than FIL, with a higher incidence of AEs.
Keywords: Janus kinase inhibitors; advanced therapy; filgotinib; inflammatory bowel disease; tofacitinib; ulcerative colitis; upadacitinib.
© 2024 The Author(s). United European Gastroenterology Journal published by Wiley Periodicals LLC on behalf of United European Gastroenterology.
Conflict of interest statement
Kunio Asonuma has served as an endowed chair from AbbVie, JIMRO, Zeria Pharmaceutical, Kyorin Pharmaceutical, Mochida Pharmaceutical, and EA Pharma; and has served as a speaker of EA Pharma, Mitsubishi Tanabe Pharma, Takeda, and AbbVie GK. Shinji Okabayashi has received speaking fees from Mitsubishi Tanabe Pharma and consulting fees from EA Pharma. Maiko Ikenouchi has received speaking fees from Mochida Pharmaceutical, and Mitsubishi Tanabe Pharma. Shinichiro Shinzaki has received lecture or consulting fees from AbbVie GK, EA Pharma Co. Ltd., Eli Lilly Japan K.K., Gilead Sciences, Inc., Janssen Pharmaceutical K.K., JIMRO Co. Ltd., Kissei Pharmaceutical Co., Ltd., Kyorin Pharmaceutical Co. Ltd., Mitsubishi Tanabe Pharma Corporation, Mochida Pharmaceutical Co., Ltd., Nippon Kayaku Co. Ltd., Pfizer Inc., Sekisui Medical Co., Ltd., and Takeda Pharmaceutical Co. Ltd., and research grant and scholarship grant from AbbVie GK, EA Pharma Co. Ltd., JIMRO Co. Ltd., Kyorin Pharmaceutical Co. Ltd., Nippon Kayaku Co. Ltd., Mochida Pharmaceutical Co., Ltd, Zeria Pharmaceutical Co. Ltd. Masayuki Fukata has received speaking fees from Takeda Pharmaceutical Co. Ltd., Janssen Pharmaceutical K.K., Kyorin Pharmaceutical, Zeria Pharmaceutical, AbbVie GK, Kissei Pharmaceutical, Pfizer Japan Inc., Daiichi Sankyo Co. Ltd., Sekisui Medical, and EA Pharma. Taku Kobayashi has served as an advisory board member, consultant, or speaker for AbbVie, Activaid, Alfresa Pharma, Alimentiv, Bristol Myers Squibb, Celltrion, Covidien, EA Pharma, Eli Lilly Japan K.K., Ferring Pharmaceuticals, Galapagos, Gilead Sciences, Janssen Pharmaceuticals, JIMRO, Kissei Pharmaceutical, Kyorin Pharmaceutical, Mitsubishi Tanabe Pharma, Mochida Pharmaceutical, Nippon Kayaku, Pfizer, Takeda, and Zeria Pharmaceutical, and has received research funding from AbbVie, Alfresa Pharma, EA Pharma, Gilead Sciences, Kyorin Pharmaceutical, Mochida Pharmaceutical, Nippon Kayaku, Otsuka Holdings, Pfizer, Sekisui Medical, Takeda, and Zeria Pharmaceutical. None of the funding received for this work was from any of the above organizations. The authors not listed have no conflicts of interest to declare.
Figures
References
-
- Lasa J. S., Olivera P. A., Danese S., and Peyrin‐Biroulet L., “Efficacy and Safety of Biologics and Small Molecule Drugs for Patients With Moderate‐to‐Severe Ulcerative Colitis: A Systematic Review and Network Meta‐Analysis,” Lancet Gastroenterol Hepatol 7, no. 2 (2022): 161–170, 10.1016/S2468-1253(21)00377-0. - DOI - PubMed
-
- Danese S., Vermeire S., Zhou W., et al., “Upadacitinib as Induction and Maintenance Therapy for Moderately to Severely Active Ulcerative Colitis: Results from Three Phase 3, Multicentre, Double‐Blind, Randomised Trials,” Lancet 399, no. 10341 (2022): 2113–2128, 10.1016/S0140-6736(22)00581-5. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

