Managing Urticarial Vasculitis: A Clinical Decision-Making Algorithm Based on Expert Consensus
- PMID: 39535577
- PMCID: PMC11748462
- DOI: 10.1007/s40257-024-00902-y
Managing Urticarial Vasculitis: A Clinical Decision-Making Algorithm Based on Expert Consensus
Abstract
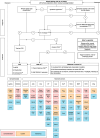
Urticarial vasculitis (UV) is a rare and difficult-to-treat, small-vessel leukocytoclastic vasculitis presenting with recurrent long-lasting wheals. So far, no guidelines and treatment algorithms exist that could help clinicians with the management of UV. In this review, we describe evidence on systemic treatments used for UV and propose a clinical decision-making algorithm for UV management based on the Urticarial Vasculitis Activity Score assessed for 7 days (UVAS7). Patients with occasional UV-like urticarial lesions and patients with UV with skin-limited manifestations and/or mild arthralgia/malaise (total UVAS7 ≤7 of 70) can be initially treated using the step-wise algorithm for chronic urticaria including second-generation H1-antihistamines, omalizumab, and cyclosporine A. Patients with UV with more severe symptoms (UVAS7 >7), especially those with hypocomplementemic UV, may require a multidisciplinary approach, particularly if underlying diseases, for example, systemic lupus erythematosus, cancer, or infection, are present. Immunomodulatory therapy is based on clinical signs and symptoms, and the drug availability and safety profile, and includes systemic corticosteroids, dapsone, hydroxychloroquine, anti-interleukin-1 agents, and other therapies. The level of evidence for all UV treatments is low. Prospective studies with current and novel drugs are needed and could provide further insights into UV pathogenesis and treatment.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Funding: Open Access funding enabled and organized by Projekt DEAL. Conflicts of Interest/Competing Interests: Nikolai Dario Rothermel, Carolina Vera Ayala, Leonie Shirin Herzog, Polina Pyatilova, and Sophia Neisinger have no conflicts of interest that are directly relevant to the content of this article. Emek Kocatürk was a speaker/consultant and/or advisor for and/or has received research funding from Novartis, Menarini, LaRoche Posey, Sanofi, Bayer, Abdi İbrahim, and Pfizer outside of the submitted work. Indrashis Podder has no conflicts of interest to declare in relation to the current work. Outside of it, he is or recently was a speaker and/or advisor for Menarini, Sun Pharmaceuticals, Glenmark, Cipla, and Alkem Laboratories. Jie Shen Fok has previously received speaker honorarium or/and travel sponsorship from CSL Behring, Menarini, Viatris, Takeda, and Novartis outside of submitted work. Manuel P. Pereira has received research funding from Almirall and Pfizer; is an investigator for Allakos, Celldex Therapeutics, Incyte, Sanofi, and Trevi Therapeutics; and has received consulting fees, speaker honoraria and/or travel fees from AbbVie, Beiersdorf, Celltrion, Doctorflix, Eli Lilly, GA2LEN, Galderma, Menlo Therapeutics, Novartis, P.G. Unna Academy, Sanofi, Streamed UP, and Trevi Therapeutics. Margarida Gonçalo is or has been advisor and/or received fees for lectures from AbbVie, Astra-Zeneca, Leo Pharma, Lilly, Novartis, Pfizer, Sanofi, and Takeda outside of the submitted work. Melba Munoz is or recently was, outside of the submitted work, a speaker and/or advisor for and/or has received research funding from Jasper Therapeutics, Celldex Therapeutics, Takeda, GA2LEN, UNEV, Astra Zeneca, and Roche. Karoline Krause has no conflict of interest related to this work. Outside of it, she received research funding and or honoraria from Bayer, Beiersdorf, Berlin Chemie, CSL Behring, Moxie, Novartis, Roche/CHUGAI, Sobi, and Takeda. Marcus Maurer is or recently was, outside of the submitted work, a speaker and/or advisor for and/or has received research funding from Allakos, Alexion, Alvotech, Almirall, Amgen, Aquestive, argenX, AstraZeneca, Celldex, Celltrion, Clinuvel, Escient, Evommune, Excellergy, GSK, Incyte, Jasper, Kashiv, Kyowa Kirin, Leo Pharma, Lilly, Menarini, Mitsubishi Tanabe Pharma, Moxie, Noucor, Novartis, Orion Biotechnology, Resoncance Medicine, Sanofi/Regeneron, Santa Ana Bio, Septerna, Servier, Third HarmonicBio, ValenzaBio, Vitalli Bio, Yuhan Corporation, and Zurabio. Hanna Bonnekoh was a speaker/consultant and/or advisor for and/or has received research funding AbbVie, Novartis, Sanofi Aventis, and ValenzaBio outside of the submitted work. Pavel Kolkhir was a speaker/consultant and/or advisor for and/or has received research funding from Novartis, ValenzaBio, and Roche outside of the submitted work. Ethics Approval: Not applicable. Consent to Participate: Not applicable. Consent for Publication: Not applicable. Availability of Data and Material: This is a review article and does not involve human subjects, therefore no primary data were used. Data from studies presented in this article are summarized in the tables and supplementary tables. Code Availability: Not applicable. Authors’ Contributions: NR, HB, and PK contributed to the conceptualization, methodology, data curation, and writing (original draft). AR, CVA, EK, IP, JF, LH, MP, MG, MMu, PP, and SN contributed to the methodology, data curation, and writing (original draft). MM and KK contributed to data curation and writing (review and editing). All authors contributed to the consensus process that led to this article, reviewed the literature, critically reviewed and revised the article, and proofread and approved the final version.
Figures
Similar articles
-
Management of urticarial vasculitis: A worldwide physician perspective.World Allergy Organ J. 2020 Mar 5;13(3):100107. doi: 10.1016/j.waojou.2020.100107. eCollection 2020 Mar. World Allergy Organ J. 2020. PMID: 32180892 Free PMC article.
-
Treatment of urticarial vasculitis: A systematic review.J Allergy Clin Immunol. 2019 Feb;143(2):458-466. doi: 10.1016/j.jaci.2018.09.007. Epub 2018 Sep 27. J Allergy Clin Immunol. 2019. PMID: 30268388
-
Urticarial vasculitis.Allergy Asthma Proc. 2007 Jan-Feb;28(1):97-100. doi: 10.2500/aap.2007.28.2972. Allergy Asthma Proc. 2007. PMID: 17390766 Review.
-
Urticarial vasculitis.Curr Opin Rheumatol. 2025 Jan 1;37(1):45-50. doi: 10.1097/BOR.0000000000001058. Epub 2024 Oct 11. Curr Opin Rheumatol. 2025. PMID: 39600289 Review.
-
Urticarial vasculitis.Clin Rev Allergy Immunol. 2002 Oct;23(2):201-16. doi: 10.1385/CRIAI:23:2:201. Clin Rev Allergy Immunol. 2002. PMID: 12221865 Review.
Cited by
-
Urticarial Vasculitis: A Case Report of Comorbid Generalized Anxiety Disorder.Cureus. 2025 Jun 26;17(6):e86802. doi: 10.7759/cureus.86802. eCollection 2025 Jun. Cureus. 2025. PMID: 40718197 Free PMC article.
-
Challenging Clinical Therapeutic Approach to Urticarial Vasculitis: A Case Series.J Clin Med. 2025 Jun 27;14(13):4580. doi: 10.3390/jcm14134580. J Clin Med. 2025. PMID: 40648952 Free PMC article.
References
-
- Kolkhir P, Grakhova M, Bonnekoh H, Krause K, Maurer M. Treatment of urticarial vasculitis: s systematic review. J Allergy Clin Immunol. 2019;143(2):458–66. 10.1016/j.jaci.2018.09.007. - PubMed
-
- Bonnekoh H, Jelden-Thurm J, Butze M, Krause K, Maurer M, Kolkhir P. In urticarial vasculitis, long disease duration, high symptom burden, and high need for therapy are linked to low patient-reported quality of life. J Allergy Clin Immunol Pract. 2022;10(10):2734-41.e7. 10.1016/j.jaip.2022.07.003. - PubMed
-
- Krause K, Mahamed A, Weller K, Metz M, Zuberbier T, Maurer M. Efficacy and safety of canakinumab in urticarial vasculitis: an open-label study. J Allergy Clin Immunol. 2013;132(3):751-4.e5. 10.1016/j.jaci.2013.04.008. - PubMed
-
- Bonnekoh H, Jelden-Thurm J, Allenova A, Chen Y, Cherrez-Ojeda I, Danilycheva I, et al. Urticarial vasculitis differs from chronic spontaneous urticaria in time to diagnosis, clinical presentation, and need for anti-inflammatory treatment: an international prospective UCARE study. J Allergy Clin Immunol Pract. 2023;11(9):2900-10.e21. 10.1016/j.jaip.2023.06.030. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

