Comparison of the dosimetry and cell survival effect of 177Lu and 161Tb somatostatin analog radiopharmaceuticals in cancer cell clusters and micrometastases
- PMID: 39535653
- PMCID: PMC11561253
- DOI: 10.1186/s40658-024-00696-2
Comparison of the dosimetry and cell survival effect of 177Lu and 161Tb somatostatin analog radiopharmaceuticals in cancer cell clusters and micrometastases
Abstract
Background: 177Lu-based radiopharmaceuticals (RPs) are the most used for targeted radionuclide therapy (TRT) due to their good response rates. However, the worldwide availability of 177Lu is limited. 161Tb represents a potential alternative for TRT, as it emits photons for SPECT imaging, β--particles for therapy, and also releases a significant yield of internal conversion (IE) and Auger electrons (AE). This research aimed to evaluate cell dosimetry with the MIRDcell code considering a realistic localization of three 161Tb- and 177Lu-somatostatin (SST) analogs in different subcellular regions as reported in the literature, various cell cluster sizes (25-1000 µm of radius) and percentage of labeled cells. Experimental values of the α- and β-survival coefficients determined by external beam photon irradiation were used to estimate the survival fraction (SF) of AR42J pancreatic cell clusters and micrometastases.
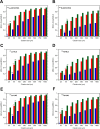
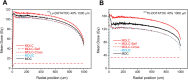
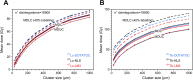
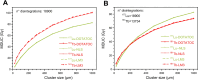
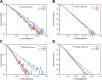
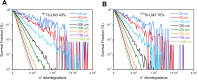
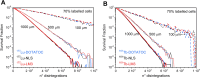
Results: The different localization of RPs labeled with the same radionuclide within the cells, resulted in only slight variations in the dose absorbed by the nuclei (ADN) of the labeled cells with no differences observed in either the unlabeled cells or the SF. ADN of labeled cells (MDLC) produced by 161Tb-RPs were from 2.8-3.7 times higher than those delivered by 177Lu-RPs in cell clusters with a radius lower than 0.1 mm and 10% of labeled cells, due to the higher amount of energy emitted by 161Tb-disintegration in form of IE and AE. However, the 161Tb-RPs/177Lu-RPs MDLC ratio decreased below 1.6 in larger cell clusters (0.5-1 mm) with > 40% labeled cells, due to the significantly higher 177Lu-RPs cross-irradiation contribution. Using a fixed number of disintegrations, SFs of 161Tb-RPs in clusters with > 40% labeled cells were lower than those of 177Lu-RPs, but when the same amount of emitted energy was used no significant differences in SF were observed between 177Lu- and 161Tb-RPs, except for the smallest cluster sizes.
Conclusions: Despite the emissions of IE and AE from 161Tb-RPs, their localization within different subcellular regions exerted a negligible influence on the ADN. The same cell damage produced by 177Lu-RPs could be achieved using smaller quantities of 161Tb-RPs, thus making 161Tb a suitable alternative for TRT.
Keywords: 161Tb; 177Lu; Cell dosimetry; Radiopharmaceuticals; Somatostatin analogs; Targeted radionuclide therapy; Theranostics.
© 2024. The Author(s).
Conflict of interest statement
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

