Non-cell autonomous regulation of cell-cell signaling and differentiation by mitochondrial ROS
- PMID: 39535785
- PMCID: PMC11561560
- DOI: 10.1083/jcb.202401084
Non-cell autonomous regulation of cell-cell signaling and differentiation by mitochondrial ROS
Abstract
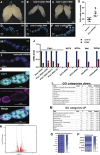
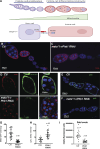
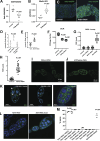
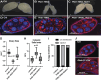
Mitochondrial reactive oxygen species (ROS) function intrinsically within cells to induce cell damage, regulate transcription, and cause genome instability. However, we know little about how mitochondrial ROS production non-cell autonomously impacts cell-cell signaling. Here, we show that mitochondrial dysfunction inhibits the plasma membrane localization of cell surface receptors that drive cell-cell communication during oogenesis. Within minutes, we found that mitochondrial ROS impairs exocyst membrane binding and leads to defective endosomal recycling. This endosomal defect impairs the trafficking of receptors, such as the Notch ligand Delta, during oogenesis. Remarkably, we found that overexpressing RAB11 restores ligand trafficking and rescues the developmental defects caused by ROS production. ROS production from adjacent cells acutely initiates a transcriptional response associated with growth and migration by suppressing Notch signaling and inducing extra cellualr matrix (ECM) remodeling. Our work reveals a conserved rapid response to ROS production that links mitochondrial dysfunction to the non-cell autonomous regulation of cell-cell signaling.
© 2024 Du et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures







Similar articles
-
Rab11 plays a key role in stellate cell differentiation via non-canonical Notch pathway in Malpighian tubules of Drosophila melanogaster.Dev Biol. 2020 May 1;461(1):19-30. doi: 10.1016/j.ydbio.2020.01.002. Epub 2020 Jan 3. Dev Biol. 2020. PMID: 31911183
-
Rab family proteins regulate the endosomal trafficking and function of RGS4.J Biol Chem. 2013 Jul 26;288(30):21836-49. doi: 10.1074/jbc.M113.466888. Epub 2013 Jun 3. J Biol Chem. 2013. PMID: 23733193 Free PMC article.
-
Numb regulates the balance between Notch recycling and late-endosome targeting in Drosophila neural progenitor cells.Mol Biol Cell. 2016 Sep 15;27(18):2857-66. doi: 10.1091/mbc.E15-11-0751. Epub 2016 Jul 27. Mol Biol Cell. 2016. PMID: 27466320 Free PMC article.
-
Ligand-Independent Mechanisms of Notch Activity.Trends Cell Biol. 2015 Nov;25(11):697-707. doi: 10.1016/j.tcb.2015.07.010. Epub 2015 Oct 1. Trends Cell Biol. 2015. PMID: 26437585 Free PMC article. Review.
-
The functional interplay of Rab11, FIP3 and Rho proteins on the endosomal recycling pathway controls cell shape and symmetry.Small GTPases. 2018 Jul 4;9(4):310-315. doi: 10.1080/21541248.2016.1224288. Epub 2016 Sep 2. Small GTPases. 2018. PMID: 27533792 Free PMC article. Review.
References
-
- Aytes, A., Mitrofanova A., Kinkade C.W., Lefebvre C., Lei M., Phelan V., LeKaye H.C., Koutcher J.A., Cardiff R.D., Califano A., et al. . 2013. ETV4 promotes metastasis in response to activation of PI3-kinase and Ras signaling in a mouse model of advanced prostate cancer. Proc. Natl. Acad. Sci. USA. 110:E3506–E3515. 10.1073/pnas.1303558110 - DOI - PMC - PubMed
-
- Ban, Y., Tan P., Cai J., Li J., Hu M., Zhou Y., Mei Y., Tan Y., Li X., Zeng Z., et al. . 2020. LNCAROD is stabilized by m6A methylation and promotes cancer progression via forming a ternary complex with HSPA1A and YBX1 in head and neck squamous cell carcinoma. Mol. Oncol. 14:1282–1296. 10.1002/1878-0261.12676 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

