Genetic and Cellular Basis of Impaired Phagocytosis and Photoreceptor Degeneration in CLN3 Disease
- PMID: 39535788
- PMCID: PMC11563035
- DOI: 10.1167/iovs.65.13.23
Genetic and Cellular Basis of Impaired Phagocytosis and Photoreceptor Degeneration in CLN3 Disease
Abstract
Purpose: CLN3 Batten disease (also known as juvenile neuronal ceroid lipofuscinosis) is a lysosomal storage disorder that typically initiates with retinal degeneration but is followed by seizure onset, motor decline and premature death. Patient-derived CLN3 disease induced pluripotent stem cell-RPE cells show defective phagocytosis of photoreceptor outer segment (POS). Because modifier genes are implicated in CLN3 disease, our goal here was to investigate a direct link between CLN3 mutation and POS phagocytosis defect.
Methods: Isogenic control and CLN3 mutant stem cell lines were generated by CRISPR-Cas9-mediated biallelic deletion of exons 7 and 8. A transgenic CLN3Δ7-8/Δ7-8 (CLN3) Yucatan miniswine was also used to study the impact of CLN3Δ7-8/Δ7-8 mutation on POS phagocytosis. POS phagocytosis by cultured RPE cells was analyzed by Western blotting and immunohistochemistry. Electroretinogram, optical coherence tomography and histological analysis of CLN3Δ7-8/Δ7-8 and wild-type miniswine eyes were carried out at 6, 36, or 48 months of age.
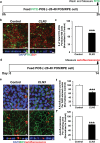
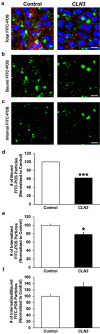
Results: CLN3Δ7-8/Δ7-8 RPE (CLN3 RPE) displayed decreased POS binding and consequently decreased uptake of POS compared with isogenic control RPE cells. Furthermore, wild-type miniswine RPE cells phagocytosed CLN3Δ7-8/Δ7-8 POS less efficiently than wild-type POS. Consistent with decreased POS phagocytosis, lipofuscin/autofluorescence was decreased in CLN3 miniswine RPE at 36 months of age and was followed by almost complete loss of photoreceptors at 48 months of age.
Conclusions: CLN3Δ7-8/Δ7-8 mutation (which affects ≤85% of patients) affects both RPE and POS and leads to photoreceptor cell loss in CLN3 disease. Furthermore, both primary RPE dysfunction and mutant POS independently contribute to impaired POS phagocytosis in CLN3 disease.
Conflict of interest statement
Disclosure:
Figures



Update of
-
Genetic and cellular basis of impaired phagocytosis and photoreceptor degeneration in CLN3 disease.bioRxiv [Preprint]. 2024 Jun 13:2024.06.09.597388. doi: 10.1101/2024.06.09.597388. bioRxiv. 2024. Update in: Invest Ophthalmol Vis Sci. 2024 Nov 4;65(13):23. doi: 10.1167/iovs.65.13.23. PMID: 38895469 Free PMC article. Updated. Preprint.
References
-
- Haltia M, Goebel HH.. The neuronal ceroid-lipofuscinoses: a historical introduction. Biochim Biophys Acta. 2013; 1832(11): 1795–1800. - PubMed
-
- Anderson GW, Goebel HH, Simonati A.. Human pathology in NCL. Biochim Biophys Acta. 2013; 1832(11): 1807–1826. - PubMed
-
- Mole SE, Williams RE, Goebel HH.. Correlations between genotype, ultrastructural morphology and clinical phenotype in the neuronal ceroid lipofuscinoses. Neurogenetics. 2005; 6(3): 107–126. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

