MID1-COMPLEMENTING ACTIVITY regulates cell proliferation and development via Ca2+ signaling in Marchantia polymorpha
- PMID: 39535860
- PMCID: PMC11663713
- DOI: 10.1093/plphys/kiae613
MID1-COMPLEMENTING ACTIVITY regulates cell proliferation and development via Ca2+ signaling in Marchantia polymorpha
Abstract
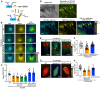
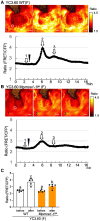
MID1-COMPLEMENTING ACTIVITY (MCA) is a land plant-specific, plasma membrane protein, and Ca2+ signaling component that responds to exogenous mechanical stimuli, such as touch, gravity, and hypotonic-osmotic stress, in various plant species. MCA is essential for cell proliferation and differentiation during growth and development in rice (Oryza sativa) and maize (Zea mays). However, the mechanism by which MCA mediates cell proliferation and differentiation via Ca2+ signaling remains unknown. Here, we address this question using the liverwort Marchantia polymorpha. We show that the M. polymorpha MCA ortholog, MpMCA, is highly expressed in actively dividing regions, such as apical notches in the thalli and developing gametangiophores, and that MpMCA is a plasma membrane protein. In vivo, Ca2+ imaging using a Ca2+ sensor (yellow cameleon) revealed that MpMCA is required for maintaining proper [Ca2+]cyt levels in the apical notch region, egg cells, and antheridium cells. Mpmca mutant plants showed severe cell proliferation and differentiation defects in the thalli, gametangiophores, and gametangia, resulting in abnormal development and unsuccessful fertilization. Furthermore, expression of the Arabidopsis MCA1 gene complemented most of the defects in the growth and development of the Mpmca mutant plants. Our findings indicate that MpMCA is an evolutionarily conserved Ca2+-signaling component that regulates cell proliferation and development across the life cycle of land plants.
© The Author(s) 2024. Published by Oxford University Press on behalf of American Society of Plant Biologists.
Conflict of interest statement
Conflict of interest statement. None declared.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

