Collaborative Care to Improve Quality of Life for Anxiety and Depression in Posttraumatic Epilepsy (CoCarePTE): Protocol for a Randomized Hybrid Effectiveness-Implementation Trial
- PMID: 39535875
- PMCID: PMC11602765
- DOI: 10.2196/59329
Collaborative Care to Improve Quality of Life for Anxiety and Depression in Posttraumatic Epilepsy (CoCarePTE): Protocol for a Randomized Hybrid Effectiveness-Implementation Trial
Abstract
Background: Anxiety and depression in people with epilepsy are common and associated with poor outcomes; yet, they often go untreated due to poor mental health specialist access. Collaborative care is an integrated care model with a strong evidence base in primary care and medical settings, but it has not been evaluated in neurology clinics. Evaluating implementation outcomes when translating evidence-based interventions to new clinical settings to inform future scaling and incorporation into real-world practice is important.
Objective: The Collaborative Care for Posttraumatic Epilepsy (CoCarePTE) trial aims to evaluate the effectiveness (improvement in emotional quality of life) and implementation of a collaborative care intervention for people with anxiety or depressive symptoms and posttraumatic epilepsy.
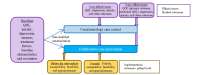
Methods: CoCarePTE is a 2-site, randomized, single-blind, hybrid type 1 effectiveness-implementation trial that will randomize 60 adults to receive either neurology-based collaborative care or usual care. Adults receiving neurological care at participating centers with anxiety or depressive symptoms and a history of at least mild traumatic brain injury before epilepsy onset will be enrolled. The collaborative care intervention is a 24-week stepped-care model with video or telephone calls every 2 weeks by a care manager for measurement-based anxiety and depression care, seizure care monitoring, and brief therapy intervention delivery. This is supplemented by antidepressant prescribing recommendations by psychiatrists for neurologists via case conferences and care manager-facilitated team communication. In step 2 of the intervention, individuals with <50% symptom reduction by 10 weeks will receive an added 8-session remote cognitive behavioral therapy program. The study is powered to detect a moderate improvement in emotional quality of life. As a hybrid type 1 trial, effectiveness is the primary focus, with the primary outcome being a change in emotional quality of life at 6 months in the intervention group compared to control. Secondary effectiveness outcomes are 6-month changes in depression, anxiety, and overall quality of life. Implementation outcomes, including fidelity, acceptability, feasibility, and appropriateness, are evaluated before implementation and at 3 months. The primary effectiveness analysis will compare changes in emotional quality of life scores from baseline to 6 months between the intervention and control arms using multiple linear regression modeling, adjusting for study site and using an intent-to-treat approach.
Results: Enrollment commenced in 2023, with modifications in the inclusion and exclusion made after the first 6 enrollees due to slow recruitment. Enrollment is expected to continue at least into early 2025.
Conclusions: The CoCarePTE trial is novel in its use of a hybrid effectiveness-implementation design to evaluate an evidence-based mental health intervention in epilepsy, and by incorporating seizure care into a collaborative care model. If a significant improvement in emotional quality of life is found in the intervention group compared to usual care, this would support next step scaling or clinical implementation.
Trial registration: ClinicalTrials.gov NCT05353452; https://www.clinicaltrials.gov/study/NCT05353452.
International registered report identifier (irrid): DERR1-10.2196/59329.
Keywords: epilepsy; integrated care; mental health; neurology clinic; psychiatric comorbidity; seizures.
©Heidi M Munger Clary, Beverly M Snively, Christian Cagle, Richard Kennerly, James N Kimball, Halley B Alexander, Gretchen A Brenes, Justin B Moore, Robin A Hurley. Originally published in JMIR Research Protocols (https://www.researchprotocols.org), 13.11.2024.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures
References
-
- Kwan P, Yu E, Leung H, Leon T, Mychaskiw MA. Association of subjective anxiety, depression, and sleep disturbance with quality-of-life ratings in adults with epilepsy. Epilepsia. 2009 May;50(5):1059–66. doi: 10.1111/j.1528-1167.2008.01938.x. http://dx.doi.org/10.1111/j.1528-1167.2008.01938.x EPI1938 - DOI - DOI - PubMed
-
- Tellez-Zenteno JF, Patten SB, Jetté N, Williams J, Wiebe S. Psychiatric comorbidity in epilepsy: a population-based analysis. Epilepsia. 2007 Dec;48(12):2336–44. doi: 10.1111/j.1528-1167.2007.01222.x. https://onlinelibrary.wiley.com/doi/10.1111/j.1528-1167.2007.01222.x EPI1222 - DOI - DOI - PubMed
-
- Fazel S, Wolf A, Långström N, Newton CR, Lichtenstein P. Premature mortality in epilepsy and the role of psychiatric comorbidity: a total population study. Lancet. 2013 Nov 16;382(9905):1646–54. doi: 10.1016/S0140-6736(13)60899-5. https://linkinghub.elsevier.com/retrieve/pii/S0140-6736(13)60899-5 S0140-6736(13)60899-5 - DOI - PMC - PubMed
-
- Caller TA, Chen JJ, Harrington JJ, Bujarski KA, Jobst BC. Predictors for readmissions after video-EEG monitoring. Neurology. 2014 Jul 29;83(5):450–5. doi: 10.1212/WNL.0000000000000647. https://europepmc.org/abstract/MED/24951478 WNL.0000000000000647 - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials