Calcium dynamics of skin-resident macrophages during homeostasis and tissue injury
- PMID: 39535893
- PMCID: PMC11656469
- DOI: 10.1091/mbc.E24-09-0420
Calcium dynamics of skin-resident macrophages during homeostasis and tissue injury
Abstract
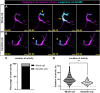
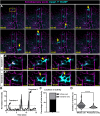
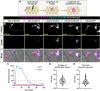
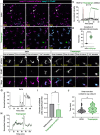
Immune cells depend on rapid changes in intracellular calcium activity to modulate cell function. Skin contains diverse immune cell types and is critically dependent on calcium signaling for homeostasis and repair, yet the dynamics and functions of calcium in skin immune cells remain poorly understood. Here, we characterize calcium activity in Langerhans cells, skin-resident macrophages responsible for surveillance and clearance of cellular debris after tissue damage. Langerhans cells reside in the epidermis and extend dynamic dendrites in close proximity to adjacent keratinocytes and somatosensory peripheral axons. We find that homeostatic Langerhans cells exhibit spontaneous and transient changes in calcium activity, with calcium flux occurring primarily in the cell body and rarely in the dendrites. Triggering somatosensory axon degeneration increases the frequency of calcium activity in Langerhans cell dendrites. By contrast, we show that Langerhans cells exhibit a sustained increase in intracellular calcium following engulfment of damaged keratinocytes. Altering intracellular calcium activity leads to a decrease in engulfment efficiency of keratinocyte debris. Our findings demonstrate that Langerhans cells exhibit context-specific changes in calcium activity and highlight the utility of skin as an accessible model for imaging calcium dynamics in tissue-resident macrophages.
Figures

Update of
-
Calcium dynamics of skin-resident macrophages during homeostasis and tissue injury.bioRxiv [Preprint]. 2024 Sep 26:2024.09.24.614510. doi: 10.1101/2024.09.24.614510. bioRxiv. 2024. Update in: Mol Biol Cell. 2024 Dec 1;35(12):br26. doi: 10.1091/mbc.E24-09-0420. PMID: 39386455 Free PMC article. Updated. Preprint.
Similar articles
-
Calcium dynamics of skin-resident macrophages during homeostasis and tissue injury.bioRxiv [Preprint]. 2024 Sep 26:2024.09.24.614510. doi: 10.1101/2024.09.24.614510. bioRxiv. 2024. Update in: Mol Biol Cell. 2024 Dec 1;35(12):br26. doi: 10.1091/mbc.E24-09-0420. PMID: 39386455 Free PMC article. Updated. Preprint.
-
Rho-associated kinase regulates Langerhans cell morphology and responsiveness to tissue damage.Cell Rep. 2024 May 28;43(5):114208. doi: 10.1016/j.celrep.2024.114208. Epub 2024 May 9. Cell Rep. 2024. PMID: 38728139
-
Zebrafish cutaneous injury models reveal that Langerhans cells engulf axonal debris in adult epidermis.Dis Model Mech. 2023 Apr 1;16(4):dmm049911. doi: 10.1242/dmm.049911. Epub 2023 Apr 3. Dis Model Mech. 2023. PMID: 36876992 Free PMC article.
-
Ontogeny and function of murine epidermal Langerhans cells.Nat Immunol. 2017 Sep 19;18(10):1068-1075. doi: 10.1038/ni.3815. Nat Immunol. 2017. PMID: 28926543 Free PMC article. Review.
-
Cytokine-mediated communication by keratinocytes and Langerhans cells with dendritic epidermal T cells.Semin Immunol. 1996 Dec;8(6):333-9. doi: 10.1006/smim.1996.0044. Semin Immunol. 1996. PMID: 8961384 Review.
Cited by
-
Microtubule-dependent cell polarity regulates skin-resident macrophage phagocytosis and directed cell migration.bioRxiv [Preprint]. 2025 Mar 14:2025.03.13.642867. doi: 10.1101/2025.03.13.642867. bioRxiv. 2025. PMID: 40161838 Free PMC article. Preprint.
References
-
- Brand A, Hovav A-H, Clausen BE (2023). Langerhans cells in the skin and oral mucosa: Brothers in arms? Eur J Immunol 53, 2149499. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

