Protective effect of mesenchymal stromal cells in diabetic nephropathy: the In vitro and In vivo role of the M-Sec-tunneling nanotubes
- PMID: 39535903
- PMCID: PMC11609313
- DOI: 10.1042/CS20242064
Protective effect of mesenchymal stromal cells in diabetic nephropathy: the In vitro and In vivo role of the M-Sec-tunneling nanotubes
Abstract
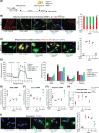
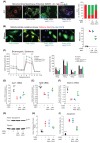
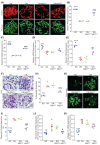
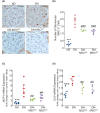
Mitochondrial dysfunction plays an important role in the development of podocyte injury in diabetic nephropathy (DN). Tunnelling nanotubes (TNTs) are long channels that connect cells and allow organelle exchange. Mesenchymal stromal cells (MSCs) can transfer mitochondria to other cells through the M-Sec-TNTs system. However, it remains unexplored whether MSCs can form heterotypic TNTs with podocytes, thereby enabling the replacement of diabetes-damaged mitochondria. In this study, we analysed TNT formation, mitochondrial transfer, and markers of cell injury in podocytes that were pre-exposed to diabetes-related insults and then co-cultured with diabetic or non-diabetic MSCs. Furthermore, to assess the in vivo relevance, we treated DN mice with exogenous MSCs, either expressing or lacking M-Sec, carrying fluorescent-tagged mitochondria. MSCs formed heterotypic TNTs with podocytes, allowing mitochondrial transfer, via a M-Sec-dependent mechanism. This ameliorated mitochondrial function, nephrin expression, and reduced apoptosis in recipient podocytes. However, MSCs isolated from diabetic mice failed to confer cytoprotection due to Miro-1 down-regulation. In experimental DN, treatment with exogenous MSCs significantly improved DN, but no benefit was observed in mice treated with MSCs lacking M-Sec. Mitochondrial transfer from exogenous MSCs to podocytes occurred in vivo in a M-Sec-dependent manner. These findings demonstrate that the M-Sec-TNT-mediated transfer of mitochondria from healthy MSCs to diabetes-injured podocytes can ameliorate podocyte damage. Moreover, M-Sec expression in exogenous MSCs is essential for providing renoprotection in vivo in experimental DN.
Keywords: albuminuria; diabetic nephropathy; mesenchymal stem cell; mitochondria; podocytes; tunneling nanotubes.
© 2024 The Author(s).
Conflict of interest statement
The Authors declares that there are no competing interests associated with the manuscript.
Figures




Similar articles
-
Protective effect of the tunneling nanotube-TNFAIP2/M-sec system on podocyte autophagy in diabetic nephropathy.Autophagy. 2023 Feb;19(2):505-524. doi: 10.1080/15548627.2022.2080382. Epub 2022 Jun 6. Autophagy. 2023. PMID: 35659195 Free PMC article.
-
Protective Role of the M-Sec-Tunneling Nanotube System in Podocytes.J Am Soc Nephrol. 2021 May 3;32(5):1114-1130. doi: 10.1681/ASN.2020071076. Epub 2021 Mar 15. J Am Soc Nephrol. 2021. PMID: 33722931 Free PMC article.
-
Podocyte RNF166 deficiency alleviates diabetic nephropathy by mitigating mitochondria impairment and apoptosis via regulation of CYLD signal.Biochem Biophys Res Commun. 2021 Mar 19;545:46-53. doi: 10.1016/j.bbrc.2020.12.014. Epub 2021 Feb 2. Biochem Biophys Res Commun. 2021. PMID: 33545631
-
Tunneling Nanotubes-Mediated Protection of Mesenchymal Stem Cells: An Update from Preclinical Studies.Int J Mol Sci. 2020 May 14;21(10):3481. doi: 10.3390/ijms21103481. Int J Mol Sci. 2020. PMID: 32423160 Free PMC article. Review.
-
Podocyte Bioenergetics in the Development of Diabetic Nephropathy: The Role of Mitochondria.Endocrinology. 2022 Jan 1;163(1):bqab234. doi: 10.1210/endocr/bqab234. Endocrinology. 2022. PMID: 34791124 Free PMC article. Review.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

