From Gas to Solution: The Changing Neutral Structure of Proline upon Solvation
- PMID: 39536145
- PMCID: PMC11613541
- DOI: 10.1021/acs.jpca.4c05628
From Gas to Solution: The Changing Neutral Structure of Proline upon Solvation
Abstract
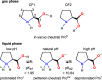
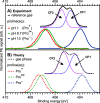
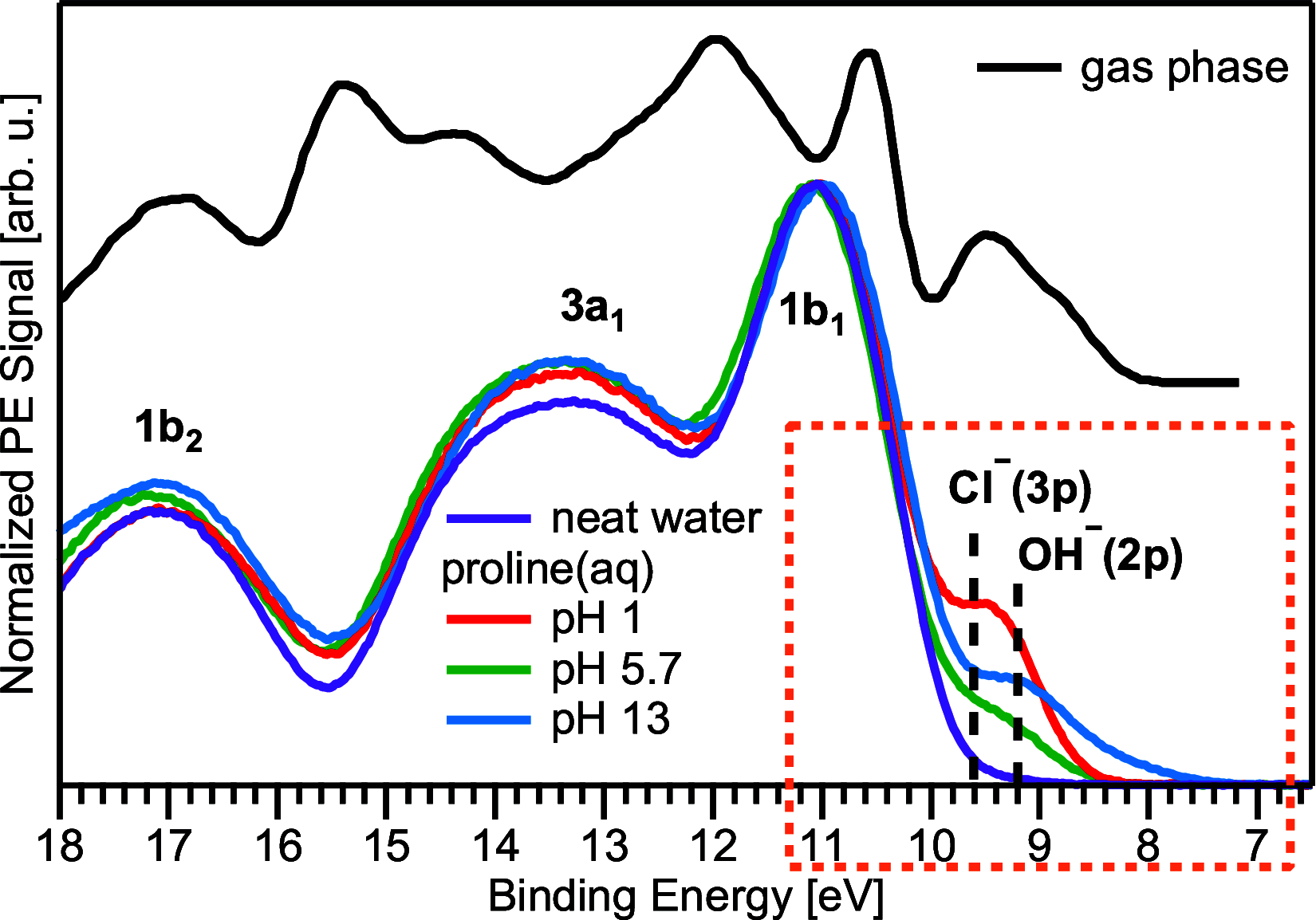
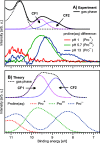
Liquid-jet photoelectron spectroscopy (LJ-PES) and electronic-structure theory were employed to investigate the chemical and structural properties of the amino acid l-proline in aqueous solution for its three ionized states (protonated, zwitterionic, and deprotonated). This is the first PES study of this amino acid in its biologically relevant environment. Proline's structure in the aqueous phase under neutral conditions is zwitterionic, distinctly different from the nonionic neutral form in the gas phase. By analyzing the carbon 1s and nitrogen 1s core levels as well as the valence spectra of aqueous-phase proline, we found that the electronic structure is dominated by the protonation state of each constituent molecular site (the carboxyl and amine groups) with small yet noticeable interference across the molecule. The site-specific nature of the core-level spectra enables the probing of individual molecular constituents. The valence photoelectron spectra are more difficult to interpret because of the overlapping signals of proline with the solvent and pH-adjusting agents (HCl and NaOH). Yet, we are able to reveal subtle effects of specific (hydrogen-bonding) interaction with the solvent on the electronic structure. We also demonstrate that the relevant conformational space is much smaller for aqueous-phase proline than for its gas-phase analogue. This study suggests that caution must be taken when comparing photoelectron spectra for gaseous- and aqueous-phase molecules, particularly if those molecules are readily protonated/deprotonated in solution.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Photoelectron circular dichroism of aqueous-phase alanine.Chem Sci. 2025 Apr 7;16(20):8637-8647. doi: 10.1039/d5sc00167f. eCollection 2025 May 21. Chem Sci. 2025. PMID: 40255964 Free PMC article.
-
Local electronic structure of histidine in aqueous solution.Phys Chem Chem Phys. 2021 Apr 14;23(14):8847-8853. doi: 10.1039/d1cp00361e. Epub 2021 Mar 31. Phys Chem Chem Phys. 2021. PMID: 33876044
-
On the origins of core-electron chemical shifts of small biomolecules in aqueous solution: insights from photoemission and ab initio calculations of glycine(aq).J Am Chem Soc. 2011 Mar 9;133(9):3120-30. doi: 10.1021/ja110321q. Epub 2011 Feb 14. J Am Chem Soc. 2011. PMID: 21319819
-
Flexible H2O2 in water: electronic structure from photoelectron spectroscopy and ab initio calculations.J Phys Chem A. 2011 Jun 16;115(23):6239-49. doi: 10.1021/jp111674s. Epub 2011 Feb 21. J Phys Chem A. 2011. PMID: 21332235
-
The Clusters-in-a-Liquid Approach for Solvation: New Insights from the Conformer Specific Gas Phase Spectroscopy and Vibrational Optical Activity Spectroscopy.Front Chem. 2016 Feb 25;4:9. doi: 10.3389/fchem.2016.00009. eCollection 2016. Front Chem. 2016. PMID: 26942177 Free PMC article. Review.
Cited by
-
Photoelectron circular dichroism of aqueous-phase alanine.Chem Sci. 2025 Apr 7;16(20):8637-8647. doi: 10.1039/d5sc00167f. eCollection 2025 May 21. Chem Sci. 2025. PMID: 40255964 Free PMC article.

