Proteomics Reveals Age as Major Modifier of Inflammatory CSF Signatures in Multiple Sclerosis
- PMID: 39536291
- PMCID: PMC11563564
- DOI: 10.1212/NXI.0000000000200322
Proteomics Reveals Age as Major Modifier of Inflammatory CSF Signatures in Multiple Sclerosis
Abstract
Background and objectives: Multiple sclerosis (MS) can start as relapsing or progressive. While their clinical features and treatment responses are distinct, it has remained uncertain whether their pathomechanisms differ. A notable age-related effect on MS phenotype and response to immunotherapies is well acknowledged, but the underlying pathophysiologic reasons are yet to be fully elucidated. We aimed to identify disease-specific and age-related proteomic signatures using a comprehensive targeted proteomic analysis.
Methods: In our retrospective cohort study, we analyzed the CSF and serum proteome of age-matched individuals with treatment-naïve relapsing-remitting and primary progressive MS, neurologic controls (NC), and individuals with neuroborreliosis using targeted proteomics and validated findings in an independent cohort. Proteomic results were integrated with clinical and laboratory covariates.
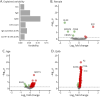
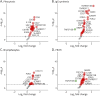
Results: Among 2,500 proteins, 47 CSF proteins were distinct between individuals with MS (n = 60) and NC (n = 20), with a subset also differing from those with neuroborreliosis (n = 8). We identified MS-associated proteins, including novel candidate biomarkers such as LY9 and JCHAIN, and putative treatment targets, such as SLAMF7, BCMA, and IL5RA, for which drugs are already licensed in other indications. The CSF proteome differences between relapsing and progressive MS were minimal, but major changes were noted in individuals older than 50 years, indicating a shift from MS-associated inflammatory to age-related protein signature. NEFL was the only serum protein that differed between individuals with MS and controls.
Discussion: This study unveils a unique CSF proteomic signature in MS, providing new pathophysiologic insights and identifying novel biomarker candidates and potential therapeutic targets. Our findings highlight similar immunologic mechanisms in relapsing and progressive MS and underscore aging's profound effect on the intrathecal immune response. This aligns with the observed lower efficacy of immunotherapies in the elderly, thus emphasizing the necessity for alternative therapeutic approaches in treating individuals with MS beyond the age of 50.
Conflict of interest statement
F. Held and C. Makarov report no disclosures relevant to the manuscript; C. Gasperi received research support from the German Federal Ministry of Education and Research (BMBF), the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation), the Hertie Foundation, and the Hans and Klementia Langmatz Stiftung; M. Flaskamp and V. Grummel report no disclosures relevant to the manuscript; A. Berthele received research support from the German Federal Ministry of Education and Research (BMBF; grant 01ZZ2102B), consulting and/or speaker fees from Alexion, Argenx, Biogen, Horizon, Novartis, Roche and Sandoz/Hexal and his institution has received compensation for clinical trials from Alexion, Biogen, Merck, Novartis, Roche, and Sanofi Genzyme, all outside the present work; B. Hemmer has served on scientific advisory boards for Novartis and Hoffmann LaRoche and has served as DMSC member for AllergyCare, Sandoz, Polpharma, Biocon, and TG therapeutics. B. Hemmer and his institution received research grants from Roche for multiple sclerosis research. B. Hemmer received honoraria for counseling (Gerson Lehrmann Group), holds part of 2 patents: one for the detection of antibodies against KIR4.1 in a subpopulation of patients with multiple sclerosis and one for genetic determinants of neutralizing antibodies to interferon. B. Hemmer is associated with DIFUTURE (Data Integration for Future Medicine) [BMBF 01ZZ1804[A-I]] and received research support from the European Union's Horizon 2020 Research and Innovation Program [grant MultipleMS, EU RIA 733161] and the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany's Excellence Strategy within the framework of the Munich Cluster for Systems Neurology [EXC 2145 SyNergy - ID 390857198]. All conflicts are not relevant to the topic of the study. Go to
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
