Riboflavin kinase binds and activates inducible nitric oxide synthase to reprogram macrophage polarization
- PMID: 39536592
- PMCID: PMC11605425
- DOI: 10.1016/j.redox.2024.103413
Riboflavin kinase binds and activates inducible nitric oxide synthase to reprogram macrophage polarization
Abstract
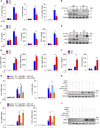
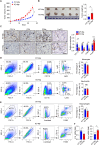
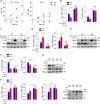
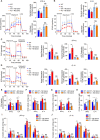
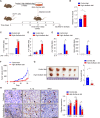
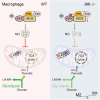
Riboflavin kinase (RFK) is essential in riboflavin metabolism, converting riboflavin to flavin mononucleotide (FMN), which is further processed to flavin adenine dinucleotide (FAD). While RFK enhances macrophage phagocytosis of Listeria monocytogenes, its role in macrophage polarization is not well understood. Our study reveals that RFK deficiency impairs M(IFN-γ) and promotes M(IL-4) polarization, both in vitro and in vivo. Mechanistically, RFK interacts with inducible nitric oxide (NO) synthase (iNOS), which requires FMN and FAD as cofactors for activation, leading to increased NO production that alters energy metabolism by inhibiting the tricarboxylic acid cycle and mitochondrial electron transport chain. Exogenous FAD reverses the metabolic and polarization changes caused by RFK deficiency. Furthermore, bone marrow adoptive transfer from high-riboflavin-fed mice into wild-type tumor-bearing mice reprograms tumor-associated macrophage polarization and inhibits tumor growth. These results suggest that targeting RFK-iNOS or modulating riboflavin metabolism could be potential therapies for macrophage-related immune diseases.
Keywords: Inducible nitric oxide synthase; Macrophage polarization; Riboflavin kinase.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Murray P.J., Allen J.E., Biswas S.K., Fisher E.A., Gilroy D.W., Goerdt S., Gordon S., Hamilton J.A., Ivashkiv L.B., Lawrence T., Locati M., Mantovani A., Martinez F.O., Mege J.L., Mosser D.M., Natoli G., Saeij J.P., Schultze J.L., Shirey K.A., Sica A., Suttles J., Udalova I., van Ginderachter J.A., Vogel S.N., Wynn T.A. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41(1):14–20. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

