Intravenous Dextrose for the Treatment of Neonatal Hypoglycaemia: A Systematic Review
- PMID: 39536722
- PMCID: PMC11965860
- DOI: 10.1159/000541471
Intravenous Dextrose for the Treatment of Neonatal Hypoglycaemia: A Systematic Review
Abstract
Introduction: Hypoglycaemic neonates are usually admitted to neonatal intensive care for intravenous (IV) dextrose infusion if increased feeding and dextrose gel fail to restore normoglycaemia. However, the effectiveness of this intervention is uncertain. This review aimed to assess the evidence for the risks and benefits of IV dextrose for treatment of neonatal hypoglycaemia.
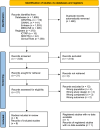
Methods: Four databases and three clinical trial registries were searched from inception to October 5, 2023. Randomised controlled trials (RCTs), non-randomised studies of interventions, cohort studies, and before and after studies were considered for inclusion without language or publication date restrictions. Risk of bias was assessed using Cochrane's Risk of Bias 2 tool or Risk of Bias in Non-Randomized Studies of Interventions tool. Certainty of evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation approach. Meta-analysis was planned but not carried out due to insufficient data.
Results: Across 6 studies (two RCTs and four cohort), 711 participants were included. Evidence from one cohort study suggests IV dextrose treatment may not be associated with neurodevelopmental impairment at ≥18 months of age (no effect numbers, p > 0.2; very low certainty evidence; 60 infants). Evidence from one RCT suggests IV dextrose treatment may reduce the likelihood of repeated hypoglycaemia (risk ratio [RR]: 0.67 [95% CI: 0.20, 2.18], p = 0.5; low certainty evidence; 80 infants) compared to treatment with oral sucrose bolus. However, the risk of a hyperglycaemic episode may be increased (RR: 2.33 [95% CI: 0.65, 8.39], p = 0.19; 80 infants).
Conclusion: More evidence is needed to clarify the benefits and risks of IV dextrose for treatment of neonatal hypoglycaemia.
Introduction: Hypoglycaemic neonates are usually admitted to neonatal intensive care for intravenous (IV) dextrose infusion if increased feeding and dextrose gel fail to restore normoglycaemia. However, the effectiveness of this intervention is uncertain. This review aimed to assess the evidence for the risks and benefits of IV dextrose for treatment of neonatal hypoglycaemia.
Methods: Four databases and three clinical trial registries were searched from inception to October 5, 2023. Randomised controlled trials (RCTs), non-randomised studies of interventions, cohort studies, and before and after studies were considered for inclusion without language or publication date restrictions. Risk of bias was assessed using Cochrane's Risk of Bias 2 tool or Risk of Bias in Non-Randomized Studies of Interventions tool. Certainty of evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation approach. Meta-analysis was planned but not carried out due to insufficient data.
Results: Across 6 studies (two RCTs and four cohort), 711 participants were included. Evidence from one cohort study suggests IV dextrose treatment may not be associated with neurodevelopmental impairment at ≥18 months of age (no effect numbers, p > 0.2; very low certainty evidence; 60 infants). Evidence from one RCT suggests IV dextrose treatment may reduce the likelihood of repeated hypoglycaemia (risk ratio [RR]: 0.67 [95% CI: 0.20, 2.18], p = 0.5; low certainty evidence; 80 infants) compared to treatment with oral sucrose bolus. However, the risk of a hyperglycaemic episode may be increased (RR: 2.33 [95% CI: 0.65, 8.39], p = 0.19; 80 infants).
Conclusion: More evidence is needed to clarify the benefits and risks of IV dextrose for treatment of neonatal hypoglycaemia.
Keywords: Blood glucose; Infant; Intravenous infusion; Newborn.
© 2024 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Oral dextrose gel to prevent hypoglycaemia in at-risk neonates.Cochrane Database Syst Rev. 2021 May 17;5(5):CD012152. doi: 10.1002/14651858.CD012152.pub3. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2023 Nov 28;11:CD012152. doi: 10.1002/14651858.CD012152.pub4. PMID: 33998668 Free PMC article. Updated.
-
Early Feeding for the Prevention of Neonatal Hypoglycaemia: A Systematic Review and Meta-Analysis.Neonatology. 2024;121(2):141-156. doi: 10.1159/000535503. Epub 2024 Jan 9. Neonatology. 2024. PMID: 38194933 Free PMC article.
-
First aid glucose administration routes for symptomatic hypoglycaemia.Cochrane Database Syst Rev. 2019 Apr 11;4(4):CD013283. doi: 10.1002/14651858.CD013283.pub2. Cochrane Database Syst Rev. 2019. PMID: 30973639 Free PMC article.
-
Skin-to-skin contact for the prevention of neonatal hypoglycaemia: a systematic review and meta-analysis.BMC Pregnancy Childbirth. 2023 Oct 21;23(1):744. doi: 10.1186/s12884-023-06057-8. BMC Pregnancy Childbirth. 2023. PMID: 37865757 Free PMC article.
-
Intrapartum maternal glycaemic control for the prevention of neonatal hypoglycaemia: a systematic review and meta-analysis.BMC Pregnancy Childbirth. 2024 Jun 13;24(1):423. doi: 10.1186/s12884-024-06615-8. BMC Pregnancy Childbirth. 2024. PMID: 38872105 Free PMC article.
References
-
- Harris D, Weston P, Gamble G, Harding J. Glucose profiles in healthy term infants in the first 5 days: the Glucose in Well babies (GLOW) study. J Pediatr. 2020;223:34–41.e4. - PubMed
-
- Harris D, Weston P, Harding J. Incidence of neonatal hypoglycemia in babies identified as at risk. J Pediatr. 2012;161(5):787–91. - PubMed
-
- Wackernagel D, Gustafsson A, Edstedt Bonamy A-K, Reims A, Ahlsson F, Elfving M, et al. . Swedish national guideline for prevention and treatment of neonatal hypoglycaemia in newborn infants with gestational age ≥35 weeks. Acta Paediatr. 2020;109(1):31–44. - PubMed
-
- Shah R, Harding J, Brown J, McKinlay C. Neonatal glycaemia and neurodevelopmental outcomes: a systematic review and meta-analysis. Neonatology. 2019;115(2):116–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical