PD-1 blockade plus cisplatin-based chemotherapy in patients with small cell/neuroendocrine bladder and prostate cancers
- PMID: 39536751
- PMCID: PMC11604497
- DOI: 10.1016/j.xcrm.2024.101824
PD-1 blockade plus cisplatin-based chemotherapy in patients with small cell/neuroendocrine bladder and prostate cancers
Abstract
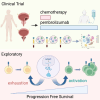
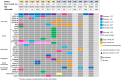
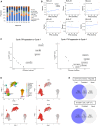
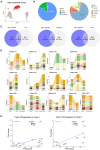
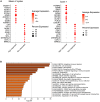
Small cell neuroendocrine cancers share biologic similarities across tissue types, including transient response to platinum-based chemotherapy with rapid progression of disease. We report a phase 1b study of pembrolizumab in combination with platinum-based chemotherapy in 15 patients with stage III-IV small cell bladder (cohort 1) or small cell/neuroendocrine prostate cancers (cohort 2). Overall response rate (ORR) is 43% with two-year overall survival (OS) rate of 86% (95% confidence interval [CI]: 0.63, 1.00) for cohort 1 and 57% (95% CI: 0.30, 1.00) for cohort 2. Treatment is tolerated well with grade 3 or higher adverse events occurring in 40% of patients with no deaths or treatment cessation secondary to toxicity. Single-cell and T cell receptor sequencing of serial peripheral blood samples reveals clonal expansion of diverse T cell repertoire correlating with progression-free survival. Our results demonstrate promising efficacy and safety of this treatment combination and support future investigation of this biomarker. This study was registered at ClinicalTrials.gov (NCT03582475).
Keywords: PD-1 blockade; T cell receptor sequencing; clinical trial; immunochemotherapy; neuroendocrine prostate cancer; single-cell sequencing; small cell bladder cancer; small cell cancers.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

