Impact of Palivizumab in Preventing Severe Acute Lower Respiratory Infection in Moderate-to-Late Preterm Infants: A Nationwide Cohort Study
- PMID: 39536788
- PMCID: PMC11557254
- DOI: 10.3346/jkms.2024.39.e279
Impact of Palivizumab in Preventing Severe Acute Lower Respiratory Infection in Moderate-to-Late Preterm Infants: A Nationwide Cohort Study
Abstract
Background: Respiratory syncytial virus (RSV) prophylaxis using palivizumab effectively reduces RSV-associated morbidity in preterm infants. In Korea, national insurance coverage for palivizumab was implemented in October 2016 for moderate-to-late preterm (MLPT) infants born during the RSV season (October-March) who have older siblings. However, no large-scale studies have investigated the changes in the incidence and risk of severe acute lower respiratory infections (ALRIs) after insurance coverage implementation for MLPT infants.
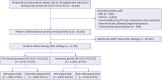
Methods: This large-scale retrospective cohort study used data from the Korean National Health Insurance Service between October 2013 and December 2019. MLPT infants (32 0/7-35 6/7 weeks of gestation) with older siblings were stratified into pre-insurance period (PIP; October 2013-September 2016) and insurance period (IP; October 2016-March 2019) groups based on the date of birth with respect to initial insurance palivizumab implementation. Severe ALRI outcomes (hospitalization, respiratory support, and intensive care unit admission) were evaluated up to 1 year of age using multivariable logistic regression models.
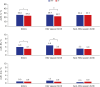
Results: Of the 11,722 MLPT infants included in the study, 6,716 and 5,006 infants were included in the IP and PIP groups, respectively. The incidences of ALRI-hospitalization and ALRI-respiratory support were significantly lower in the IP group than that in PIP group (24.0% vs. 26.0% and 3.1% vs. 4.0%, respectively). Additionally, ALRI-respiratory support risk was significantly lower in the IP group (adjusted odds ratio 0.771, 95% confidence interval 0.626-0.949, P = 0.014) than that in the PIP group. Among infants born during the RSV season, the risk of ALRI-hospitalization and ALRI-respiratory support were significantly lower in the IP group than that in the PIP group. However, no significant differences were observed between the IP and PIP groups for infants born during the non-RSV season.
Conclusion: The risks of severe ALRI outcomes decreased in Korea following the 2016 insurance implementation of palivizumab prophylaxis for MLPT infants born during the RSV season with older siblings.
Keywords: Infant, Premature; Palivizumab; Prophylaxis; Respiratory Syncytial Virus; Respiratory Tract Infection.
© 2024 The Korean Academy of Medical Sciences.
Conflict of interest statement
The authors have no potential conflicts of interest to disclose.
Figures

Similar articles
-
Palivizumab coverage rates among moderate-to-late preterm infants in Korea: a nationwide cross-sectional study.Epidemiol Health. 2025;47:e2025015. doi: 10.4178/epih.e2025015. Epub 2025 Apr 1. Epidemiol Health. 2025. PMID: 40211817 Free PMC article.
-
Efficacy of palivizumab prophylaxis on the frequency of RSV-associated lower respiratory tract infections in preterm infants: determination of the ideal target population for prophylaxis.Eur J Clin Microbiol Infect Dis. 2017 Sep;36(9):1629-1634. doi: 10.1007/s10096-017-2976-x. Epub 2017 Apr 8. Eur J Clin Microbiol Infect Dis. 2017. PMID: 28391538
-
Impact of the 2014 American Academy of Pediatrics recommendation and of the resulting limited financial coverage by the Italian Medicines Agency for palivizumab prophylaxis on the RSV-associated hospitalizations in preterm infants during the 2016-2017 epidemic season: a systematic review of seven Italian reports.Ital J Pediatr. 2019 Nov 9;45(1):139. doi: 10.1186/s13052-019-0736-5. Ital J Pediatr. 2019. PMID: 31706338 Free PMC article.
-
Global disease burden of and risk factors for acute lower respiratory infections caused by respiratory syncytial virus in preterm infants and young children in 2019: a systematic review and meta-analysis of aggregated and individual participant data.Lancet. 2024 Mar 30;403(10433):1241-1253. doi: 10.1016/S0140-6736(24)00138-7. Epub 2024 Feb 14. Lancet. 2024. PMID: 38367641
-
Respiratory Syncytial Virus Burden in Premature Infants: The Role of Season With and Without RSV Immunoprophylaxis in a Multicenter Study.Pediatr Pulmonol. 2025 Mar;60(3):e71022. doi: 10.1002/ppul.71022. Pediatr Pulmonol. 2025. PMID: 40042149 Free PMC article.
Cited by
-
Palivizumab coverage rates among moderate-to-late preterm infants in Korea: a nationwide cross-sectional study.Epidemiol Health. 2025;47:e2025015. doi: 10.4178/epih.e2025015. Epub 2025 Apr 1. Epidemiol Health. 2025. PMID: 40211817 Free PMC article.
References
-
- Frey HA, Klebanoff MA. The epidemiology, etiology, and costs of preterm birth. Semin Fetal Neonatal Med. 2016;21(2):68–73. - PubMed
-
- Smyth RL, Openshaw PJ. Bronchiolitis. Lancet. 2006;368(9532):312–322. - PubMed
-
- Li Y, Wang X, Blau DM, Caballero MT, Feikin DR, Gill CJ, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet. 2022;399(10340):2047–2064. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

