Examining downstream effects of concizumab in hemophilia A with a mathematical modeling approach
- PMID: 39536817
- PMCID: PMC12336664
- DOI: 10.1016/j.jtha.2024.10.028
Examining downstream effects of concizumab in hemophilia A with a mathematical modeling approach
Abstract
Background: Tissue factor (TF) pathway inhibitor (TFPI) is an anticoagulant protein that inhibits factor (F)Xa, the TF-FVIIa-FXa complex, and early forms of the prothrombinase complex. Concizumab is a monoclonal antibody that blocks FXa inhibition by TFPI and reduces bleeding in hemophilia.
Objectives: To examine how concizumab impacts various reactions of TFPI to restore thrombin generation in hemophilia A using mathematical models.
Methods: A compartment model was used to estimate plasma concentrations of free concizumab and its complexes with TFPIα and TFPIβ. Concizumab was integrated into a flow-mediated mathematical model of coagulation, and a small injury was simulated under hemophilia A conditions. Simulations were then analyzed to determine how concizumab's blockade of TFPI anticoagulant activities, specifically the inhibition of FXa in plasma and on platelets, inhibition of TF:FVIIa at the subendothelium, and prior sequestration of plasma TFPIα to the endothelium via TFPIβ, altered thrombin generation.
Results: Concizumab improved simulated thrombin generation in hemophilia A by simultaneously altering all 3 mechanisms of the TFPI anticoagulant blockade examined. Concizumab sequestered ∼75% of plasma TFPIα through the formation of ternary TFPIα-concizumab-TFPIβ-complexes. For all TF levels, reducing the TFPIα plasma concentration had the largest impact on the lag time, followed by blocking TFPIα inhibition of TF:FVIIa:FXa and subsequently by blocking TFPIα inhibition of FXa in plasma and on the platelet surface.
Conclusion: The effectiveness of concizumab is mediated through the blockade of TFPI anticoagulant activities in plasma and on multiple physiological surfaces. An important and previously unrecognized function of concizumab was the sequestration of plasma TFPIα to the endothelium.
Keywords: TFPI-antibody; concizumab; hemophilia A; mathematical modeling.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interests A.E.M. receives research funding from Novo Nordisk and Pharmacosmos and has received honoraria for serving on Novo Nordisk Advisory Boards. A.R.W., M.D., and M.K. are employees of Novo Nordisk. M.K. is a minor shareholder of Novo Nordisk. K.M. and K.L. received research funding from Novo Nordisk.
Figures

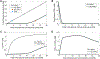


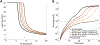

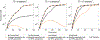
References
-
- Blanchette VS, Key NS, Ljung LR, Manco-Johnson MJ, van den Berg HM, Srivastava A, Subcommittee on Factor VIII. Factor IX and Rare Coagulation Disorders of the Scientific and Standardization Committee of the International Society on Thrombosis and Hemostasis. Definitions in hemophilia: communication from the SSC of the ISTH. J Thromb Haemost. 2014;12:1935–9. - PubMed
-
- Collins PW, Blanchette VS, Fischer K, Björkman S, Oh M, Fritsch S, Schroth P, Spotts G, Astermark J, Ewenstein B, rAHF-PFM Study Group. Break-through bleeding in relation to predicted factor VIII levels in patients receiving prophylactic treatment for severe hemophilia A. J Thromb Haemost. 2009;7:413–20. - PubMed
-
- Plug I, van der Bom JG, Peters M, Mauser-Bunschoten EP, de Goede-Bolder A, Heijnen L, Smit C, Zwart-van Rijkom JE, Willemse J, Rosendaal FR. Thirty years of hemophilia treatment in the Netherlands, 1972–2001. Blood. 2004;104:3494–500. - PubMed
-
- Oldenburg J Optimal treatment strategies for hemophilia: achievements and limitations of current prophylactic regimens. Blood. 2015;125:2038–44. - PubMed
-
- Den Uijl IEM, Mauser Bunschoten EP, Roosendaal G, Schutgens REG, Biesma DH, Grobbee DE, Fischer K. Clinical severity of haemophilia A: does the classification of the 1950s still stand? Haemophilia. 2011;17:849–53. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

