Variable glucagon metabolic actions in diverse mouse models of obesity and type 2 diabetes
- PMID: 39536823
- PMCID: PMC11617456
- DOI: 10.1016/j.molmet.2024.102064
Variable glucagon metabolic actions in diverse mouse models of obesity and type 2 diabetes
Abstract
Objective: The study aimed to investigate the effects of glucagon on metabolic pathways in mouse models of obesity, fatty liver disease, and type 2 diabetes (T2D) to determine the extent and variability of hepatic glucagon resistance in these conditions.
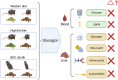
Methods: We investigated glucagon's effects in mouse models of fatty liver disease, obesity, and type 2 diabetes (T2D), including male BKS-db/db, high-fat diet-fed, and western diet-fed C57Bl/6 mice. Glucagon tolerance tests were performed using the selective glucagon receptor agonist acyl-glucagon (IUB288). Blood glucose, serum and liver metabolites include lipids and amino acids were measured. Additionally, liver protein expression related to glucagon signalling and a comprehensive liver metabolomics were performed.
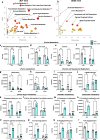
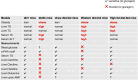
Results: Western diet-fed mice displayed impaired glucagon response, with reduced blood glucose and PKA activation. In contrast, high-fat diet-fed and db/db mice maintained normal glucagon sensitivity, showing significant elevations in blood glucose and phospho-PKA motif protein expression. Acyl-glucagon treatment also lowered liver alanine and histidine levels in high-fat diet-fed mice, but not in western diet-fed mice. Additionally, some amino acids, such as methionine, were increased by acyl-glucagon only in chow diet control mice. Despite normal glucagon sensitivity in PKA signalling, db/db mice had a distinct metabolomic response, with acyl-glucagon significantly altering 90 metabolites in db/+ mice but only 42 in db/db mice, and classic glucagon-regulated metabolites, such as cyclic adenosine monophosphate (cAMP), being less responsive in db/db mice.
Conclusions: The study reveals that hepatic glucagon resistance in obesity and T2D is complex and not uniform across metabolic pathways, underscoring the complexity of glucagon action in these conditions.
Keywords: Acylcarnitine; Amino acids; Diabetes; Glucagon; Obesity.
Copyright © 2024 The Author(s). Published by Elsevier GmbH.. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Adam Rose reports financial support was provided by Diabetes Australia. Adam Rose reports financial support was provided by National Health and Medical Research Council. Yuqin Wu reports financial support was provided by Australian Physiological Society. Adam Rose reports a relationship with Boehringer Ingelheim Pharma GmbH & Co KG that includes: funding grants. Patricia Rusu reports a relationship with Boehringer Ingelheim Pharma GmbH & Co KG that includes: funding grants. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





References
-
- Finan B., Yang B., Ottaway N., Smiley D.L., Ma T., Clemmensen C., et al. A rationally designed monomeric peptide triagonist corrects obesity and diabetes in rodents. Nat Med. 2015;21(1):27–36. - PubMed
-
- Sanyal A.J., Bedossa P., Fraessdorf M., Neff G.W., Lawitz E., Bugianesi E., et al. A phase 2 randomized trial of survodutide in MASH and fibrosis. N Engl J Med. 2024;391(4):311–319. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

