Dietary Eicosapentaenoic Acid Improves Ozone-Induced Pulmonary Inflammation in C57BL/6 Mice
- PMID: 39536972
- PMCID: PMC11867137
- DOI: 10.1016/j.tjnut.2024.11.006
Dietary Eicosapentaenoic Acid Improves Ozone-Induced Pulmonary Inflammation in C57BL/6 Mice
Abstract
Background: Ambient concentrations of the air pollutant, ozone, are rising with increasing global temperatures. Ozone is known to increase incidence and exacerbation of chronic lung diseases, which will increase as ambient ozone levels rise. Studies have identified diet as a variable that is able to modulate the pulmonary health effects associated with ozone exposure. Eicosapentaenoic acid (EPA) is an n-3 (ω-3) PUFA consumed through diet, which lowers inflammation through conversion to oxylipins including hydroxy-eicosapentaenoic acids (HEPEs). However, the role of dietary EPA in ozone-induced pulmonary inflammation is unknown.
Objective: Therefore, we hypothesized increasing dietary EPA will decrease ozone-induced pulmonary inflammation and injury through the production of HEPEs.
Methods: To test this, male C57BL/6J mice were fed a purified control diet or EPA-supplemented diet for 4 wk and then exposed to filtered air or 1 part per million ozone for 3 h. 24 or 48 h after exposure, bronchoalveolar lavage fluid was collected to assess airspace inflammation/injury and lung tissue was collected for targeted liquid chromatography-mass spectrometry lipidomics.
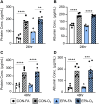
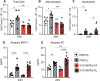
Results: Following ozone exposure, EPA supplementation did not alter markers of lung injury but decreased ozone-induced airspace neutrophilia. Targeted liquid chromatography-mass spectrometry lipidomics revealed dietary EPA supplementation increased pulmonary EPA-derived metabolites including 5-HEPE and 12-HEPE. Additionally, EPA supplementation decreased pulmonary amounts of proinflammatory arachidonic acid-derived metabolites. To evaluate whether dietary EPA reduces ozone-induced pulmonary inflammation through increased pulmonary HEPEs, C57BL/6J mice were administered 5-HEPEs and 12-HEPEs systemically before filtered air or ozone exposure. Pretreatment with 5-HEPEs and 12-HEPEs reduced ozone-driven increases in airspace macrophages.
Conclusions: Together, these data indicate that an EPA-supplemented diet protects against ozone-induced airspace inflammation which is, in part, due to increasing pulmonary amounts of 5-HEPEs and 12-HEPEs. These findings suggest that dietary EPA and/or increasing EPA-derived metabolites in the lung can reduce ozone-driven incidences and exacerbations of chronic pulmonary diseases.
Keywords: EPA; eicosapentaenoic acid; inflammation; lipid metabolism; lung; nutrition; omega-3; ozone; polyunsaturated fatty acids.
Copyright © 2024 American Society for Nutrition. Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Bae S., Lim Y.H., Oh J., Kwon H.J. Mediation of daily ambient ozone concentration on association between daily mean temperature and mortality in 7 metropolitan cities of Korea. Environ. Int. 2023;178 - PubMed
-
- Sandstrom T., Blomberg A., Bosson J.A. Can elderly lungs cope with urban concentrations of ground-level ozone? Experiences from a large-scale multicenter exposure chamber study. Am. J. Respir. Crit. Care Med. 2018;197:1245–1246. - PubMed
-
- Climate change may worsen summertime ozone pollution [Internet] 2014. https://www.nsf.gov/news/ Available from. news_summ.jsp?cntn_id=131307#:∼:text=Overall%2C%20the%20study%20found%20that,to%2079%20ppb%20at%20present.
-
- How climate change may impact ozone pollution and public health through the 21st century. United States Environmental Protection Agency; 2022. https://www.epa.gov/sciencematters/how-climate-change-may-impact-ozone-p... Available from:
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

