Cost-effectiveness of implementing a genotype-guided de-escalation strategy in patients with acute coronary syndrome
- PMID: 39537191
- PMCID: PMC12046577
- DOI: 10.1093/ehjcvp/pvae087
Cost-effectiveness of implementing a genotype-guided de-escalation strategy in patients with acute coronary syndrome
Abstract
Aims: A genotype-guided P2Y12-inhibitor de-escalation strategy, switching acute coronary syndrome (ACS) patients without a CYP2C19 loss-of-function allele from ticagrelor or prasugrel to clopidogrel, has shown to reduce bleeding risk without affecting the effectivity of therapy by increasing ischaemic risk. We estimated the cost-effectiveness of this personalized approach compared to standard dual antiplatelet therapy (DAPT; aspirin plus ticagrelor/prasugrel) in the Netherlands.
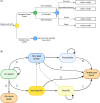
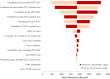
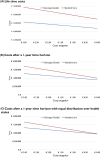
Methods and results: We developed a 1-year decision tree based on results of the FORCE-ACS registry, comparing a cohort of ACS patients who underwent genotyping with a cohort of ACS patients treated with standard DAPT. This was followed by a lifelong Markov model to compare lifetime costs and quality-adjusted life years (QALYs) for a fictional cohort of 1000 patients. The cost-effectiveness analysis was performed from the perspective of the Dutch healthcare system. A genotype-guided de-escalation strategy led to an increase of 57.73 QALYs and saved €808788 compared to standard DAPT based on a lifetime horizon. Probabilistic sensitivity analysis showed that the genotype-guided strategy was cost-saving in 96% and increased QALYs in 87% of simulations. The intervention remained cost-effective in the scenario where prices for all P2Y12 inhibitors were equalized. The genotype-guided strategy remained dominant in various other scenarios and sensitivity analyses.
Conclusion: A genotype-guided de-escalation strategy in patients with ACS was both cost-saving and yielded higher QALYs compared to standard DAPT, highlighting its potential for implementation in clinical practice. Trial registration: ClinicalTrials.gov identifier: NCT03823547.
Keywords: ACS; Coronary artery disease; Cost-effectiveness; Genotype-guided; P2Y12-inhibitor.
© The Author(s) 2024. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures

Similar articles
-
CYP2C19 LOF and GOF-Guided Antiplatelet Therapy in Patients with Acute Coronary Syndrome: A Cost-Effectiveness Analysis.Cardiovasc Drugs Ther. 2017 Feb;31(1):39-49. doi: 10.1007/s10557-016-6705-y. Cardiovasc Drugs Ther. 2017. PMID: 27924429
-
Cost-Effectiveness of Strategies to Personalize the Selection of P2Y12 Inhibitors in Patients with Acute Coronary Syndrome.Cardiovasc Drugs Ther. 2019 Oct;33(5):533-546. doi: 10.1007/s10557-019-06896-8. Cardiovasc Drugs Ther. 2019. PMID: 31367811
-
Economic Evaluations of CYP2C19 Genotype-Guided Antiplatelet Therapy Compared to the Universal Use of Antiplatelets in Patients With Acute Coronary Syndrome: A Systematic Review.J Cardiovasc Pharmacol Ther. 2020 May;25(3):201-211. doi: 10.1177/1074248420902298. Epub 2020 Feb 6. J Cardiovasc Pharmacol Ther. 2020. PMID: 32027168
-
Cost-effectiveness of CYP2C19-guided antiplatelet therapy in patients with acute coronary syndrome and percutaneous coronary intervention informed by real-world data.Pharmacogenomics J. 2020 Oct;20(5):724-735. doi: 10.1038/s41397-020-0162-5. Epub 2020 Feb 11. Pharmacogenomics J. 2020. PMID: 32042096 Free PMC article.
-
Updated Expert Consensus Statement on Platelet Function and Genetic Testing for Guiding P2Y12 Receptor Inhibitor Treatment in Percutaneous Coronary Intervention.JACC Cardiovasc Interv. 2019 Aug 26;12(16):1521-1537. doi: 10.1016/j.jcin.2019.03.034. Epub 2019 Jun 12. JACC Cardiovasc Interv. 2019. PMID: 31202949 Review.
References
-
- Byrne RA, Rossello X, Coughlan JJ, Barbato E, Berry C, Chieffo A, Claeys MJ, Dan G-A, Dweck MR, Galbraith M, Gilard M, Hinterbuchner L, Jankowska EA, Jüni P, Kimura T, Kunadian V, Leosdottir M, Lorusso R, Pedretti RFE, Rigopoulos AG, Rubini Gimenez M, Thiele H, Vranckx P, Wassmann S, Wenger NK, Ibanez B, Halvorsen S, James S, Abdelhamid M, Aboyans V, Marsan NA, Antoniou S, Asteggiano R, Bäck M, Capodanno D, Casado-Arroyo R, Cassese S, Čelutkienė J, Cikes M, Collet J-P, Ducrocq G, Falk V, Fauchier L, Geisler T, Gorog DA, Holmvang L, Jaarsma T, Jones HW, Køber L, Koskinas KC, Kotecha D, Krychtiuk KA, Landmesser U, Lazaros G, Lewis BS, Lindahl B, Linhart A, Løchen M-L, Mamas MA, Mcevoy JW, Mihaylova B, Mindham R, Mueller C, Neubeck L, Niebauer J, Nielsen JC, Niessner A, Paradies V, Pasquet AA, Petersen SE, Prescott E, Rakisheva A, Rocca B, Rosano GMC, Sade LE, Schiele F, Siller-Matula JM, Sticherling C, Storey RF, Thielmann M, Vrints C, Windecker S, Wiseth R, Witkowski A, El Amine Bouzid M, Hayrapetyan H, Metzler B, Lancellotti P, Bajrić M, Karamfiloff K, Mitsis A, Ostadal P, Sørensen R, Elwasify T, Marandi T, Ryödi E, Collet J-P, Chukhrukidze A, Mehilli J, Davlouros P, Becker D, Guðmundsdóttir IJ, Crowley J, Abramowitz Y, Indolfi C, Sakhov O, Elezi S, Beishenkulov M, Erglis A, Moussallem N, Benlamin H, Dobilienė O, Degrell P, Balbi MM, Grosu A, Lakhal Z, Ten Berg J, Pejkov H, Angel K, Witkowski A, De Sousa Almeida M, Chioncel O, Bertelli L, Stojkovic S, Studenčan M, Radšel P, Ferreiro JL, Ravn-Fischer A, Räber L, Marjeh MYB, Hassine M, Yildirir A, Parkhomenko A, Banning AP, Prescott E, James S, Arbelo E, Baigent C, Borger MA, Buccheri S, Ibanez B, Køber L, Koskinas KC, Mcevoy JW, Mihaylova B, Mindham R, Neubeck L, Nielsen JC, Pasquet AA, Rakisheva A, Rocca B, Rossello X, Vaartjes I, Vrints C, Witkowski A, Zeppenfeld K. 2023 ESC guidelines for the management of acute coronary syndromes. Eur Heart J 2023. - PubMed
-
- Capodanno D, Mehran R, Krucoff MW, Baber U, Bhatt DL, Capranzano P, Collet J-P, Cuisset T, De Luca G, De Luca L, Farb A, Franchi F, Gibson CM, Hahn J-Y, Hong M-K, James S, Kastrati A, Kimura T, Lemos PA, Lopes RD, Magee A, Matsumura R, Mochizuki S, O'donoghue ML, Pereira NL, Rao SV, Rollini F, Shirai Y, Sibbing D, Smits PC, Steg PG, Storey RF, Ten Berg J, Valgimigli M, Vranckx P, Watanabe H, Windecker S, Serruys PW, Yeh RW, Morice M-C, Angiolillo DJ. Defining strategies of modulation of antiplatelet therapy in patients with coronary artery disease: a consensus document from the Academic Research Consortium. Circulation 2023;147:1933–1944. - PubMed
-
- Gorog DA, Ferreiro JL, Ahrens I, Ako J, Geisler T, Halvorsen S, Huber K, Jeong Y-H, Navarese EP, Rubboli A, Sibbing D, Siller-Matula JM, Storey RF, Tan JWC, Ten Berg JM, Valgimigli M, Vandenbriele C, Lip GYH. De-escalation or abbreviation of dual antiplatelet therapy in acute coronary syndromes and percutaneous coronary intervention: a Consensus Statement from an international expert panel on coronary thrombosis. Nat Rev Cardiol 2023;20:830–844. - PubMed
-
- Claassens DMF, Vos GJA, Bergmeijer TO, Hermanides RS, Van ’T Hof AWJ, Van Der Harst P, Barbato E, Morisco C, Tjon Joe Gin RM, Asselbergs FW, Mosterd A, Herrman J-PR, Dewilde WJM, Janssen PWA, Kelder JC, Postma MJ, De Boer A, Boersma C, Deneer VHM, Ten Berg JM. A genotype-guided strategy for oral P2Y 12 inhibitors in primary PCI. N Engl J Med 2019;381:1621–1631. - PubMed
-
- Lee CR, Luzum JA, Sangkuhl K, Gammal RS, Sabatine MS, Stein CM, Kisor DF, Limdi NA, Lee YM, Scott SA, Hulot J‐S, Roden DM, Gaedigk A, Caudle KE, Klein TE, Johnson JA, Shuldiner AR. Clinical Pharmacogenetics Implementation Consortium Guideline for CYP2C19 genotype and clopidogrel therapy: 2022 update. Clin Pharmacol Ther 2022;112:959–967. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

