Simultaneous Protein Quantitation and Glycosylation Profiling of Antigen-Specific Immunoglobulin G1 in Large Clinical Studies
- PMID: 39537390
- PMCID: PMC11629375
- DOI: 10.1021/acs.jproteome.4c00538
Simultaneous Protein Quantitation and Glycosylation Profiling of Antigen-Specific Immunoglobulin G1 in Large Clinical Studies
Abstract
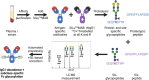
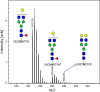
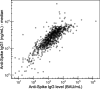
Antibodies have a key role in the immune system, making their characterization essential to biomedical, biopharmaceutical, and clinical research questions. Antibody effector functions are mainly controlled by quantity, subclass, and Fc glycosylation. We describe an integrated method to measure these three critical dimensions simultaneously. The subclass-specific immunoglobulin G (IgG) Fc glycosylation analysis combines immunosorbance with glycopeptide-centered LC-MS detection. For integrated IgG1-specific quantitation, a commercial, stable isotope labeled IgG1 protein standard was spiked into the immunosorbent eluates. Robust quantitation was achieved, relying on a combination of a proteotypic peptide and the most abundant glycopeptides, generated through proteolytic cleavage from a mixture of natural IgG1 and the recombinant IgG1 standard. Method performance was demonstrated in a large coronavirus vaccination cohort at a throughput of 100 samples/day. LC-MS-derived, anti-SARS-CoV-2 spike protein IgG1 concentrations ranged from 100 to 10000 ng/mL and correlated well with a clinically relevant immunoassay. Technical variation was 200 times lower than biological variation; intermediate precision was 44%. In conclusion, we present a method capable of robustly and simultaneously assessing quantity, subclass, and Fc glycosylation of antigen-specific IgG in large clinical studies. This method will facilitate a broader understanding of immune responses, especially the important interplay among the three dimensions.
Keywords: Glycoproteomics; LC-MS; antibody glycosylation; antibody quantitation; antigen-specific antibody responses; glycopeptides; immunoglobulin G; liquid chromatography−mass spectrometry; stable isotope labeled protein standard.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

