Management of Post-transplant Infections in Collaborating Hospitals (MATCH) Programme: a prospective cohort of all transplant recipients at Copenhagen University Hospital-Rigshospitalet, Denmark
- PMID: 39537569
- PMCID: PMC11574425
- DOI: 10.1136/bmjopen-2024-089966
Management of Post-transplant Infections in Collaborating Hospitals (MATCH) Programme: a prospective cohort of all transplant recipients at Copenhagen University Hospital-Rigshospitalet, Denmark
Abstract
Purpose: The Management of Post-transplant Infections in Collaborating Hospitals (MATCH) programme, initiated in 2011 and still ongoing, was created to 1) optimise the implementation of existing preventive strategies against viral infections in solid organ transplant (SOT) recipients and allogenic haematopoietic stem-cell transplant (HSCT) recipients and 2) advance research in the field of transplantation by collecting data from a multitude of sources.
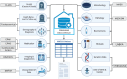
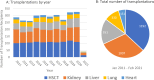
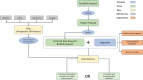
Participants: All SOT and HSCT recipients at Copenhagen University Hospital, Rigshospitalet, are followed in MATCH. By February 2021, a total of 1192 HSCT recipients and 2039 SOT recipients have been included. Participants are followed life long. An automated electronic data capture system retrieves prospective data from nationwide registries. Data from the years prior to transplantation are also collected.
Findings to date: Data entries before and after transplantation include the following: biochemistry: 13 995 222 and 26 127 817; microbiology, cultures: 242 023 and 410 558; other microbiological analyses: 265 007 and 566 402; and pathology: 170 884 and 200 394. There are genomic data on 2431 transplant recipients, whole blood biobank samples from 1003 transplant recipients and faeces biobank samples from 207 HSCT recipients. Clinical data collected in MATCH have contributed to 50 scientific papers published in peer-reviewed journals and have demonstrated success in reducing cytomegalovirus disease in SOT recipients. The programme has established international collaborations with the Swiss Transplant Cohort Study and the lung transplant cohort at Toronto General Hospital.
Future plans: Enrolment into MATCH is ongoing with no planned end date for enrolment or follow-up. MATCH will continue to provide high-quality data on transplant recipients and expand and strengthen international collaborations.
Keywords: Bone marrow transplantation; EPIDEMIOLOGY; IMMUNOLOGY; INFECTIOUS DISEASES; TRANSPLANT MEDICINE; VIROLOGY.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Ugeskriftet.dk Nyretransplantation i Danmark. 2023. https://ugeskriftet.dk/videnskab/nyretransplantation-i-danmark Available.
MeSH terms
LinkOut - more resources
Full Text Sources
