A vagus nerve dominant tetra-synaptic ascending pathway for gastric pain processing
- PMID: 39537596
- PMCID: PMC11561356
- DOI: 10.1038/s41467-024-54056-w
A vagus nerve dominant tetra-synaptic ascending pathway for gastric pain processing
Abstract
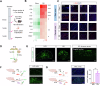
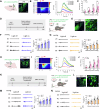
Gastric pain has limited treatment options and the mechanisms within the central circuitry remain largely unclear. This study investigates the central circuitry in gastric pain induced by noxious gastric distension (GD) in mice. Here, we identified that the nucleus tractus solitarius (NTS) serves as the first-level center of gastric pain, primarily via the vagus nerve. The prelimbic cortex (PL) is engaged in the perception of gastric pain. The lateral parabrachial nucleus (LPB) and the paraventricular thalamic nucleus (PVT) are crucial regions for synaptic transmission from the NTS to the PL. The glutamatergic tetra-synaptic NTS-LPB-PVT-PL circuitry is necessary and sufficient for the processing of gastric pain. Overall, our finding reveals a glutamatergic tetra-synaptic NTS-LPB-PVT-PL circuitry that transmits gastric nociceptive signaling by the vagus nerve in mice. It provides an insight into the gastric pain ascending pathway and offers potential therapeutic targets for relieving visceral pain.
© 2024. The Author(s).
Conflict of interest statement
Figures






References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

