Post-translational toxin modification by lactate controls Staphylococcus aureus virulence
- PMID: 39537625
- PMCID: PMC11561239
- DOI: 10.1038/s41467-024-53979-8
Post-translational toxin modification by lactate controls Staphylococcus aureus virulence
Abstract
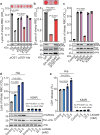
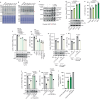
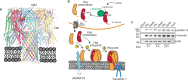
Diverse post-translational modifications have been shown to play important roles in regulating protein function in eukaryotes. By contrast, the roles of post-translational modifications in bacteria are not so well understood, particularly as they relate to pathogenesis. Here, we demonstrate post-translational protein modification by covalent addition of lactate to lysine residues (lactylation) in the human pathogen Staphylococcus aureus. Lactylation is dependent on lactate concentration and specifically affects alpha-toxin, in which a single lactylated lysine is required for full activity and virulence in infection models. Given that lactate levels typically increase during infection, our results suggest that the pathogen can use protein lactylation as a mechanism to increase toxin-mediated virulence during infection.
© 2024. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

