PPP1R2 stimulates protein phosphatase-1 through stabilisation of dynamic subunit interactions
- PMID: 39537675
- PMCID: PMC11561318
- DOI: 10.1038/s41467-024-54256-4
PPP1R2 stimulates protein phosphatase-1 through stabilisation of dynamic subunit interactions
Abstract
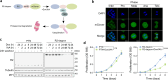
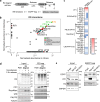
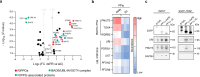
Protein Ser/Thr phosphatase PP1 is always associated with one or two regulatory subunits or RIPPOs. One of the earliest evolved RIPPOs is PPP1R2, also known as Inhibitor-2. Since its discovery nearly 5 decades ago, PPP1R2 has been variously described as an inhibitor, activator or (metal) chaperone of PP1, but it is still unknown how PPP1R2 affects the function of PP1 in intact cells. Here, using specific research tools, we demonstrate that PPP1R2 stabilises a subgroup of PP1 holoenzymes, exemplified by PP1:RepoMan, thereby promoting the dephosphorylation of their substrates. Mechanistically, the recruitment of PPP1R2 disrupts an inhibitory, fuzzy interaction between the C-terminal tail and catalytic domain of PP1, and generates an additional C-terminal RepoMan-interaction site. The resulting holoenzyme is further stabilized by a direct PPP1R2:RepoMan interaction, which renders it refractory to competitive disruption by RIPPOs that do not interact with PPP1R2. Our data demonstrate that PPP1R2 modulates the function of PP1 by altering the balance between holoenzymes through stabilisation of specific subunit interactions.
© 2024. The Author(s).
Conflict of interest statement
Figures






References
-
- Brautigan, D. L. & Shenolikar, S. Protein serine/threonine phosphatases: keys to unlocking regulators and substrates. Annu. Rev. Biochem.87, 921–964 (2018). - PubMed
-
- Egloff, M. P., Cohen, P. T. W., Reinemer, P. & Barford, D. Crystal structure of the catalytic subunit of human protein phosphatase 1 and its complex with tungstate. J. Mol. Biol.254, 942–959 (1995). - PubMed
-
- Goldberg, J. et al. Three-dimensional structure of the catalytic subunit of protein serine/threonine phosphatase-1. Nature376, 745–753 (1995). - PubMed
-
- Heroes, E. et al. The PP1 binding code: a molecular-lego strategy that governs specificity. FEBS J.280, 584–595 (2013). - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

