In vivo evaluation of complex polyps with endoscopic optical coherence tomography and deep learning during routine colonoscopy: a feasibility study
- PMID: 39537775
- PMCID: PMC11561322
- DOI: 10.1038/s41598-024-78891-5
In vivo evaluation of complex polyps with endoscopic optical coherence tomography and deep learning during routine colonoscopy: a feasibility study
Abstract
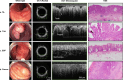
Standard-of-care (SoC) imaging for assessing colorectal polyps during colonoscopy, based on white-light colonoscopy (WLC) and narrow-band imaging (NBI), does not have sufficient accuracy to assess the invasion depth of complex polyps non-invasively during colonoscopy. We aimed to evaluate the feasibility of a custom endoscopic optical coherence tomography (OCT) probe for assessing colorectal polyps during routine colonoscopy. Patients referred for endoscopic treatment of large colorectal polyps were enrolled in this pilot clinical study, which used a side-viewing OCT catheter developed for use with an adult colonoscope. OCT images of polyps were captured during colonoscopy immediately before SoC treatment. A deep learning model was trained to differentiate benign from deeply invasive lesions for real-time diagnosis. 35 polyps from 32 patients were included. OCT imaging added on average 3:40 min (range 1:54-8:20) to the total procedure time. No complications due to OCT were observed. OCT revealed distinct subsurface tissue structures that correlated with histological findings, including tubular adenoma (n = 20), tubulovillous adenoma (n = 10), sessile serrated polyps (n = 3), and invasive cancer (n = 2). The deep learning model achieved an area under the receiver operating characteristic curve (AUROC) of 0.984 (95%CI 0.972-0.996) and Cohen's kappa of 0.845 (95%CI 0.774-0.915) when compared to gold standard histopathology. OCT is feasible and safe for polyp assessment during routine colonoscopy. When combined with deep learning, OCT offers clinicians increase confidence in identifying deeply invasive cancers, potentially improving clinical decision-making. Compared to previous studies, ours offers a nuanced comparison between not just benign and malignant lesions, but across multiple histological subtypes of polyps.
Keywords: Colonoscopy; Colorectal cancer; Deep learning; In vivo; Optical coherence tomography; Polyp.
© 2024. The Author(s).
Conflict of interest statement
Figures



Similar articles
-
Narrow band imaging versus conventional white light colonoscopy for the detection of colorectal polyps.Cochrane Database Syst Rev. 2012 Jan 18;1(1):CD008361. doi: 10.1002/14651858.CD008361.pub2. Cochrane Database Syst Rev. 2012. PMID: 22258983 Free PMC article.
-
Narrow Band Imaging, Magnifying Chromoendoscopy, and Gross Morphological Features for the Optical Diagnosis of T1 Colorectal Cancer and Deep Submucosal Invasion: A Systematic Review and Meta-Analysis.Am J Gastroenterol. 2017 Jan;112(1):54-64. doi: 10.1038/ajg.2016.403. Epub 2016 Sep 20. Am J Gastroenterol. 2017. PMID: 27644737
-
Polyp detection with colonoscopy assisted by the GI Genius artificial intelligence endoscopy module compared with standard colonoscopy in routine colonoscopy practice (COLO-DETECT): a multicentre, open-label, parallel-arm, pragmatic randomised controlled trial.Lancet Gastroenterol Hepatol. 2024 Oct;9(10):911-923. doi: 10.1016/S2468-1253(24)00161-4. Epub 2024 Aug 14. Lancet Gastroenterol Hepatol. 2024. PMID: 39153491 Clinical Trial.
-
Diagnosis of sessile serrated adenomas/polyps with image-enhanced endoscopy: a systematic review and meta-analysis.Endoscopy. 2016 Aug;48(8):731-9. doi: 10.1055/s-0042-107592. Epub 2016 May 25. Endoscopy. 2016. PMID: 27223636
-
Computer-aided diagnosis for the resect-and-discard strategy for colorectal polyps: a systematic review and meta-analysis.Lancet Gastroenterol Hepatol. 2024 Nov;9(11):1010-1019. doi: 10.1016/S2468-1253(24)00222-X. Epub 2024 Sep 17. Lancet Gastroenterol Hepatol. 2024. PMID: 39303733
Cited by
-
Deep learning for the detection of colon polyps with malignant potential: ex vivo classification using feature-enhanced optical coherence tomography (OCT) images.Biomed Opt Express. 2025 May 30;16(6):2543-2554. doi: 10.1364/BOE.555185. eCollection 2025 Jun 1. Biomed Opt Express. 2025. PMID: 40677387 Free PMC article.
References
-
- Siegel, R. L., Miller, K. D., Fuchs, H. E. & Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin.72, 7–33 (2022). - PubMed
-
- Rex, D. K., Shaukat, A. & Wallace, M. B. Optimal management of malignant polyps, from endoscopic assessment and resection to decisions about surgery. Clin. Gastroenterol. Hepatol.17, 1428–1437 (2019). - PubMed
-
- Shaukat, A. et al. Endoscopic recognition and management strategies for malignant colorectal polyps: recommendations of the US multi-society task force on colorectal cancer. Gastrointest. Endosc.92, 997–1015e1 (2020). - PubMed
-
- Lamm, V., Yu, M. A., Ciorba, M. A. & Kushnir, V. M. Not so smart? Artificial intelligence may need to go deeper to predict colorectal cancer invasion depth. Gastroenterology. 162, 1769–1770 (2022). - PubMed
-
- Angarita, F. A., Feinberg, A. E., Feinberg, S. M., Riddell, R. H. & McCart, J. A. Management of complex polyps of the colon and rectum. Int. J. Colorectal Dis.33, 115–129 (2018). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

