Widespread co-release of glutamate and GABA throughout the mouse brain
- PMID: 39537846
- PMCID: PMC11560972
- DOI: 10.1038/s42003-024-07198-y
Widespread co-release of glutamate and GABA throughout the mouse brain
Abstract
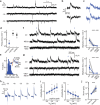
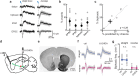
Several brain neuronal populations transmit both the excitatory and inhibitory neurotransmitters, glutamate, and GABA. However, it remains largely unknown whether these opposing neurotransmitters are co-released simultaneously or are independently transmitted at different times and locations. By recording from acute mouse brain slices, we observed biphasic miniature postsynaptic currents, i.e., minis with time-locked excitatory and inhibitory currents, in striatal spiny projection neurons. This observation cannot be explained by accidental coincidence of monophasic excitatory and inhibitory minis. Interestingly, these biphasic minis could either be an excitatory current leading an inhibitory current or vice versa. Deletion of dopaminergic neurons did not eliminate biphasic minis, indicating that they originate from another source. Importantly, we found that both types of biphasic minis were present in multiple striatal neuronal types and in nine out of ten other brain regions. Overall, co-release of glutamate and GABA appears to be a widespread mode of neurotransmission in the brain.
© 2024. The Author(s).
Conflict of interest statement
Figures



Update of
-
Prevalent co-release of glutamate and GABA throughout the mouse brain.bioRxiv [Preprint]. 2024 Mar 29:2024.03.27.587069. doi: 10.1101/2024.03.27.587069. bioRxiv. 2024. Update in: Commun Biol. 2024 Nov 13;7(1):1502. doi: 10.1038/s42003-024-07198-y. PMID: 38585864 Free PMC article. Updated. Preprint.
Similar articles
-
Prevalent co-release of glutamate and GABA throughout the mouse brain.bioRxiv [Preprint]. 2024 Mar 29:2024.03.27.587069. doi: 10.1101/2024.03.27.587069. bioRxiv. 2024. Update in: Commun Biol. 2024 Nov 13;7(1):1502. doi: 10.1038/s42003-024-07198-y. PMID: 38585864 Free PMC article. Updated. Preprint.
-
Impaired striatal D2 receptor function leads to enhanced GABA transmission in a mouse model of DYT1 dystonia.Neurobiol Dis. 2009 Apr;34(1):133-45. doi: 10.1016/j.nbd.2009.01.001. Epub 2009 Jan 13. Neurobiol Dis. 2009. PMID: 19187797 Free PMC article.
-
Neonatal nicotine exposure increases excitatory synaptic transmission and attenuates nicotine-stimulated GABA release in the adult rat hippocampus.Neuropharmacology. 2015 Jan;88:187-98. doi: 10.1016/j.neuropharm.2014.06.010. Epub 2014 Jun 17. Neuropharmacology. 2015. PMID: 24950455 Free PMC article.
-
Influence of glutamate and GABA transport on brain excitatory/inhibitory balance.Exp Biol Med (Maywood). 2021 May;246(9):1069-1083. doi: 10.1177/1535370221989263. Epub 2021 Feb 7. Exp Biol Med (Maywood). 2021. PMID: 33554649 Free PMC article. Review.
-
Functional implications of neurotransmitter co-release: glutamate and GABA share the load.Curr Opin Pharmacol. 2006 Feb;6(1):114-9. doi: 10.1016/j.coph.2005.12.001. Epub 2005 Dec 15. Curr Opin Pharmacol. 2006. PMID: 16359920 Review.
Cited by
-
Latexin and calretinin together define a novel excitatory neuron subclass in the claustrum of the short-tailed fruit bat, Carollia perspicillata.Ann N Y Acad Sci. 2025 Jun;1548(1):126-136. doi: 10.1111/nyas.15346. Epub 2025 Apr 27. Ann N Y Acad Sci. 2025. PMID: 40289365
-
Hidden in the white matter: Current views on interstitial white matter neurons.Neuroscientist. 2025 Aug;31(4):381-408. doi: 10.1177/10738584241282969. Epub 2024 Oct 4. Neuroscientist. 2025. PMID: 39365761 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
- R01NS127013/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- R01 NS127013/NS/NINDS NIH HHS/United States
- RF1 MH130784/MH/NIMH NIH HHS/United States
- RF1MH130784/U.S. Department of Health & Human Services | NIH | National Institute of Mental Health (NIMH)
- R01NS104944/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

