Adult skull bone marrow is an expanding and resilient haematopoietic reservoir
- PMID: 39537918
- PMCID: PMC11618084
- DOI: 10.1038/s41586-024-08163-9
Adult skull bone marrow is an expanding and resilient haematopoietic reservoir
Abstract
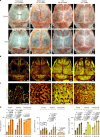
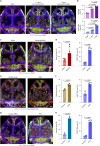
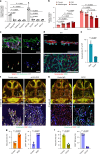
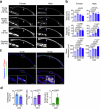
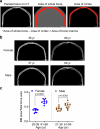
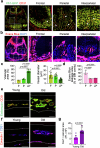
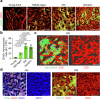
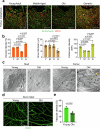
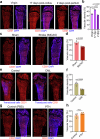
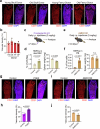
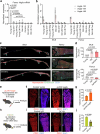
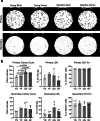
The bone marrow microenvironment is a critical regulator of haematopoietic stem cell self-renewal and fate1. Although it is appreciated that ageing, chronic inflammation and other insults compromise bone marrow function and thereby negatively affect haematopoiesis2, it is not known whether different bone compartments exhibit distinct microenvironmental properties and functional resilience. Here we use imaging, pharmacological approaches and mouse genetics to uncover specialized properties of bone marrow in adult and ageing skull. Specifically, we show that the skull bone marrow undergoes lifelong expansion involving vascular growth, which results in an increasing contribution to total haematopoietic output. Furthermore, skull is largely protected against major hallmarks of ageing, including upregulation of pro-inflammatory cytokines, adipogenesis and loss of vascular integrity. Conspicuous rapid and dynamic changes to the skull vasculature and bone marrow are induced by physiological alterations, namely pregnancy, but also pathological challenges, such as stroke and experimental chronic myeloid leukaemia. These responses are highly distinct from femur, the most extensively studied bone marrow compartment. We propose that skull harbours a protected and dynamically expanding bone marrow microenvironment, which is relevant for experimental studies and, potentially, for clinical treatments in humans.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures






References
-
- Hoggatt, J., Kfoury, Y. & Scadden, D. T. Hematopoietic stem cell niche in health and disease. Annu. Rev. Pathol.11, 555–581 (2016). - PubMed
-
- van den Bos, T., Speijer, D., Bank, R. A., Bromme, D. & Everts, V. Differences in matrix composition between calvaria and long bone in mice suggest differences in biomechanical properties and resorption: Special emphasis on collagen. Bone43, 459–468 (2008). - PubMed
-
- Sivaraj, K. K. & Adams, R. H. Blood vessel formation and function in bone. Development143, 2706–2715 (2016). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

