Intravenous and intracranial GD2-CAR T cells for H3K27M+ diffuse midline gliomas
- PMID: 39537919
- PMCID: PMC11735388
- DOI: 10.1038/s41586-024-08171-9
Intravenous and intracranial GD2-CAR T cells for H3K27M+ diffuse midline gliomas
Erratum in
-
Author Correction: Intravenous and intracranial GD2-CAR T cells for H3K27M+ diffuse midline gliomas.Nature. 2024 Dec;636(8043):E6. doi: 10.1038/s41586-024-08452-3. Nature. 2024. PMID: 39613972 Free PMC article. No abstract available.
Abstract
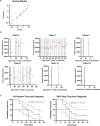
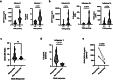
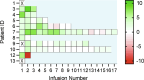
H3K27M-mutant diffuse midline gliomas (DMGs) express high levels of the disialoganglioside GD2 (ref. 1). Chimeric antigen receptor-modified T cells targeting GD2 (GD2-CART) eradicated DMGs in preclinical models1. Arm A of Phase I trial no. NCT04196413 (ref. 2) administered one intravenous (IV) dose of autologous GD2-CART to patients with H3K27M-mutant pontine (DIPG) or spinal DMG (sDMG) at two dose levels (DL1, 1 × 106 kg-1; DL2, 3 × 106 kg-1) following lymphodepleting chemotherapy. Patients with clinical or imaging benefit were eligible for subsequent intracerebroventricular (ICV) intracranial infusions (10-30 × 106 GD2-CART). Primary objectives were manufacturing feasibility, tolerability and the identification of maximally tolerated IV dose. Secondary objectives included preliminary assessments of benefit. Thirteen patients enroled, with 11 receiving IV GD2-CART on study (n = 3 DL1 (3 DIPG); n = 8 DL2 (6 DIPG, 2 sDMG)). GD2-CART manufacture was successful for all patients. No dose-limiting toxicities occurred on DL1, but three patients experienced dose-limiting cytokine release syndrome on DL2, establishing DL1 as the maximally tolerated IV dose. Nine patients received ICV infusions, with no dose-limiting toxicities. All patients exhibited tumour inflammation-associated neurotoxicity, safely managed with intensive monitoring and care. Four patients demonstrated major volumetric tumour reductions (52, 54, 91 and 100%), with a further three patients exhibiting smaller reductions. One patient exhibited a complete response ongoing for over 30 months since enrolment. Nine patients demonstrated neurological benefit, as measured by a protocol-directed clinical improvement score. Sequential IV, followed by ICV GD2-CART, induced tumour regressions and neurological improvements in patients with DIPG and those with sDMG.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: M.M., R.M. and C. Mackall are coinventors on a patent for the use of GD2-CAR T cells in regard to H2K27M gliomas, coordinated through Stanford University. C. Mackall is a coinventor on patents for the use of dasatinib and other small molecules to modulate CAR function and control CAR-associated toxicity, and on several patents related to CAR T cell therapies. C. Mackall holds equity in CARGO Therapeutics, Link Cell Therapies and GBM Newco, which are developing CAR-based therapies, and consults for CARGO, Link, Immatics, Ensoma, GBM NewCo and Red Tree Capital. She receives research funding from Lyell and Tune Therapeutics. R.M. is a cofounder of, and holds equity in, Link Cell Therapies and CARGO Therapeutics, and is a consultant for Lyell Immunopharma, NKarta, Arovella Pharmaceuticals, Innervate Radiopharmaceuticals, Aptorum Group, Gadeta, FATE Therapeutics (Data and Safety Monitoring Board) and Waypoint Bio. S.A.F. holds several patents in the field of cellular immunotherapy. V.B. is an investor and Director at Umoja Biopharma and Arsenal Bio. S.P.R. holds equity in Lyell Immunopharma. M.M. holds equity in MapLight Therapeutics and CARGO Therapeutics. The other authors declare no competing interests.
Figures










References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

