Dietary pattern and the corresponding gut microbiome in response to immunotherapy in Thai patients with advanced non-small cell lung cancer (NSCLC)
- PMID: 39537963
- PMCID: PMC11561170
- DOI: 10.1038/s41598-024-79339-6
Dietary pattern and the corresponding gut microbiome in response to immunotherapy in Thai patients with advanced non-small cell lung cancer (NSCLC)
Abstract
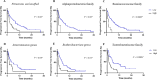
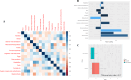
Gut microbiota is considered a key player modulating the response to immune checkpoint inhibitors (ICI) in cancer. The effects of dietary pattern on this interaction is not well-studied. A prospective multicenter cohort of 95 patients with advanced non-small cell lung cancer (NSCLC) undergoing ICI therapy were enrolled. Stool shotgun metagenomic sequencing was performed. Three-day dietary patterns before ICI were assessed. Patients were categorized as hyperprogressive disease (HPD) if they exhibited a time to treatment failure of less than 2 months. All others were categorized as non-hyperprogressive disease (non-HPD). The correlation between dietary patterns, gut microbiome, and response to ICI therapy was analyzed. In the multivariate analysis, a high abundance of Firmicutes unclassified and the Ruminococcaceae family correlated with a significantly diminished progression-free survival (PFS) with an HR of 2.40 [P = 0.006] and 4.30 [P = 0.005], respectively. More specifically, within the subset of NSCLC patients treated solely with ICI therapy, a high abundance of Intestinimonas and the Enterobacteriaceae family were associated with substantially reduced PFS with an HR of 2.61 [P = 0.02] and HR 3.34 [P = 0.005], respectively. In our comprehensive dietary pattern analysis, the HPD group showed increased consumption of cholesterol, sodium, and fats beyond recommended levels compared to the non-HPD group. This group also displayed a tendency towards higher food pattern scores characterized by a high intake of fat and dairy products. Our study revealed a distinct association between the gut microbiome composition and treatment outcomes. The overall composition of diet might be related to ICI therapeutic outcomes.
Keywords: Anti-PD-1/PD-L1 immunotherapy; Diet; Gut microbiome; Immune checkpoints inhibitors; Non-small cell lung cancer; Nutrition.
© 2024. The Author(s).
Conflict of interest statement
Figures


References
-
- Herbst, R. S. et al. Pembrolizumab versus Docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 387 (10027), 1540–1550 (2016). - PubMed
-
- Gandhi, L. et al. Pembrolizumab plus Chemotherapy in Metastatic Non-small-cell Lung Cancer. N Engl. J. Med.378 (22), 2078–2092 (2018). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

