Artemisinin-resistant Plasmodium falciparum Kelch13 mutant proteins display reduced heme-binding affinity and decreased artemisinin activation
- PMID: 39538019
- PMCID: PMC11561146
- DOI: 10.1038/s42003-024-07178-2
Artemisinin-resistant Plasmodium falciparum Kelch13 mutant proteins display reduced heme-binding affinity and decreased artemisinin activation
Abstract
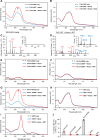
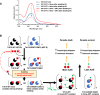
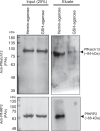
The potency of frontline antimalarial drug artemisinin (ART) derivatives is triggered by heme-induced cleavage of the endoperoxide bond to form reactive heme-ART alkoxy radicals and covalent heme-ART adducts, which are highly toxic to the parasite. ART-resistant (ART-R) parasites with mutations in the Plasmodium falciparum Kelch-containing protein Kelch13 (PfKekch13) exhibit impaired hemoglobin uptake, reduced yield of hemoglobin-derived heme, and thus decreased ART activation. However, any direct involvement of PfKelch13 in heme-mediated ART activation has not been reported. Here, we show that the purified recombinant PfKelch13 wild-type (WT) protein displays measurable binding affinity for iron and heme, the main effectors for ART activation. The heme-binding property is also exhibited by the native PfKelch13 protein from parasite culture. The two ART-R recombinant PfKelch13 mutants (C580Y and R539T) display weaker heme binding affinities compared to the ART-sensitive WT and A578S mutant proteins, which further translates into reduced yield of heme-ART derivatives when ART is incubated with the heme molecules bound to the mutant PfKelch13 proteins. In conclusion, this study provides the first evidence for ART activation via the heme-binding propensity of PfKelch13. This mechanism may contribute to the modulation of ART-R levels in malaria parasites through a novel function of PfKelch13.
© 2024. The Author(s).
Conflict of interest statement
Figures
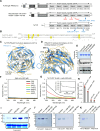
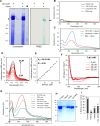
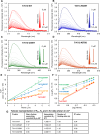



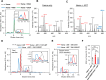
References
-
- WHO. World Health Organization. https://www.who.int. (2022).
-
- Meshnick, S. R. Artemisinin: Mechanisms of action, resistance and toxicity. Int. J. Parasitol.32, 1655–1660 (2002). - PubMed
-
- Robert, A., Coppel, Y. & Meunier, B. Alkylation of heme by the antimalarial drug artemisinin. Chem. Commun.5, 414–415 (2002). - PubMed
-
- Laurent, S. A.-L., Robert, A. & Meunier, B. C10-Modified Artemisinin Derivatives: Efficient Heme-Alkylating Agents. Angew. Chem.117, 2060–2063 (2005). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

