An Analysis of the Food and Drug Administration Manufacturer and User Facility Device Experience Database for MAGnetic Expansion Control Spinal Rods
- PMID: 39538082
- PMCID: PMC11706908
- DOI: 10.1007/s43441-024-00724-4
An Analysis of the Food and Drug Administration Manufacturer and User Facility Device Experience Database for MAGnetic Expansion Control Spinal Rods
Abstract
Background: MAGnetic Expansion Control (MAGEC) rods can prevent repeated lengthening operations for scoliosis patients. However, there have been several Field Safety Notices issued, including a worldwide product recall due to actuator endcap separation. We aimed to review adverse events reported to the Food and Drug Administration (FDA) regarding MAGEC rods, focusing on MAGEC X.
Methods: Reports submitted to the Manufacturer and User Facility Device Experience database in relation to MAGEC devices were accessed and analysed using R Statistical Software. Exclusion criteria included duplicate and literature review reports (n = 54). Free-text data were analysed using inductive content analysis.
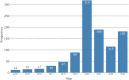
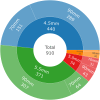
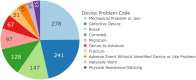
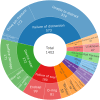
Results: 1016 adverse events were reported to 11/30/2023. 99.0% (1006) were submitted by the manufacturer. Reports primarily arose from the UK (465, 45.8%) or US (421, 41.4%). From free-text data the most frequent adverse events were distraction mechanism failure (573), device wear (272), and actuator seal damage (180). Rod fracture (n = 48) was not significantly associated with rod diameter (≤ 5.0 mm or > 5.0 mm), p = 0.736. 234 reports referenced MAGEC X devices; actuator endcap separation was identified in 41.9% (99). Other events include failure of distraction (63), surface damage (31), and rod fracture (15). On 06/30/2020 MAGEC X2 received FDA approval. Twenty reports reference devices manufactured after this date, seven describe distraction mechanism failure; notably there are no reports of endcap separation.
Conclusion: These data represent the largest series of adverse events reported for MAGEC rods, including significant new data regarding MAGEC X. As well as endcap separation, failure of distraction, surface damage, and rod fracture were reported.
Keywords: Early onset scoliosis; FDA; MAGEC; MAGEC X; MAUDE; Magnetically controlled growing rods.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing Interests: TJ undertakes expert witness work in relation to magnetic spinal rods with all monies paid to Newcastle University. JF and AB have no relevant financial or non-financial interests to disclose.
Figures


References
-
- Williams BA, Matsumoto H, McCalla DJ, Akbarnia BA, Blakemore LC, Betz RR, et al. Development and initial validation of the classification of early-onset scoliosis (C-EOS). J Bone Joint Surg Am. 2014;96(16):1359–67. 10.2106/jbjs.M.00253. - PubMed
-
- Yang S, Andras LM, Redding GJ, Skaggs DL, Early-onset scoliosis. A review of history, current treatment, and future directions. Pediatrics. 2016;137(1). 10.1542/peds.2015-0709. - PubMed
-
- Vialle LR, Berven SH, Kleuver Md. AOSpine masters series. In: Pediatric Spinal Deformities. 333 Seventh Ave. Vol. 9. New York, NY: 10001: Thieme Medical Publishers, Inc.; 2018.
-
- NuVasive. MAGEC® SYSTEM. https://www.nuvasive.com/procedures/spine/magec; 2022. Accessed 08/05/2023.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

