Anorexia nervosa-specific home treatment in children and adolescents and their families (the HoT study): a study protocol of a randomized, controlled, multicenter, open-label, parallel group superiority trial
- PMID: 39538317
- PMCID: PMC11559055
- DOI: 10.1186/s13063-024-08566-z
Anorexia nervosa-specific home treatment in children and adolescents and their families (the HoT study): a study protocol of a randomized, controlled, multicenter, open-label, parallel group superiority trial
Abstract
Background: New treatment approaches are urgently needed to improve the prognosis of children and adolescents with anorexia nervosa (AN). Recently, the feasibility of multidisciplinary home treatment that strongly involves the patients' parents/caregivers has been investigated. However, no RCT has yet been performed to test the efficacy and safety of this approach compared to standard treatment approaches, such as inpatient treatment.
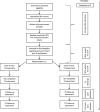
Methods: In this multicenter randomized-controlled trial, home treatment for children and adolescents with AN aged 12 to 18 years is established at 5 major treatment centers for AN in Germany. Approximately 240 patients who are admitted to the hospital for AN will be included in the trial. After a short inpatient somatic stabilization phase (5-8 weeks), patients are randomized to receive either treatment as usual (TAU), in the form of continued inpatient or day patient treatment, or the newly developed home treatment (HoT) (n = 82/arm, n = 164 in total). There are three assessments throughout treatment (admission, randomization, and discharge), as well as follow-up assessments at 9 and 12 months after admission. The BMI at 12 months after admission (primary outcome) is compared between groups (adjusted for premorbid BMI and admission BMI); secondary outcomes include eating disorder and general psychopathology, the number and duration of psychiatric rehospitalizations, quality of life, motivation for treatment and treatment satisfaction. Other secondary outcomes include the primary caregivers' burden and skills in handling the child's illness and direct treatment costs. Statistical analysis will be based on intention-to-treat principles, using mixed models for repeated measures. (Serious) adverse events are assessed throughout treatment. In addition, the feasibility and implementation of HoT as well as the satisfaction and workload of the members of the multidisciplinary treatment teams in both arms will be assessed.
Discussion: In the case of a positive evaluation, HoT can be considered an effective treatment method to replace or complete established treatment methods, such as IP, for treating AN in children and adolescents. The home treatment setting might shorten inpatient stays in this patient group, increase treatment satisfaction, and help to reduce the risk of rehospitalization, which is associated with a better outcome in this vulnerable patient group.
Trial registration: The trial was registered with the German Clinical Trial Register (DRKS) under the ID DRKS00025925 on November 26, 2021 (prospectively registered): https://drks.de/search/de/trial/DRKS00025925 .
Keywords: Adolescents; Anorexia nervosa; Health services research; Home treatment; Stepped-care.
© 2024. The Author(s).
Conflict of interest statement
Figures


References
-
- WHO, Mental health: facing the challenges, building solutions: report from the WHO European MinisterialConference. Copenhagen: World Health Organization, Regional Office for Europe; 2005.
-
- Andres-Pepina S, Plana MT, Flamarique I, Romero S, Borras R, Julia L, et al. Long-term outcome and psychiatric comorbidity of adolescent-onset anorexia nervosa. Clin Child Psychol Psychiatry. 2020;25(1):33–44. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

