Neoantigen DNA vaccines are safe, feasible, and induce neoantigen-specific immune responses in triple-negative breast cancer patients
- PMID: 39538331
- PMCID: PMC11562513
- DOI: 10.1186/s13073-024-01388-3
Neoantigen DNA vaccines are safe, feasible, and induce neoantigen-specific immune responses in triple-negative breast cancer patients
Abstract
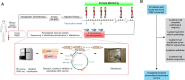
Background: Neoantigen vaccines can induce or enhance highly specific antitumor immune responses with minimal risk of autoimmunity. We have developed a neoantigen DNA vaccine platform capable of efficiently presenting both HLA class I and II epitopes and performed a phase 1 clinical trial in triple-negative breast cancer patients with persistent disease on surgical pathology following neoadjuvant chemotherapy, a patient population at high risk of disease recurrence.
Methods: Expressed somatic mutations were identified by tumor/normal exome sequencing and tumor RNA sequencing. The pVACtools software suite of neoantigen prediction algorithms was used to identify and prioritize cancer neoantigens and facilitate vaccine design for manufacture in an academic GMP facility. Neoantigen DNA vaccines were administered via electroporation in the adjuvant setting (i.e., following surgical removal of the primary tumor and completion of standard of care therapy). Vaccines were monitored for safety and immune responses via ELISpot, intracellular cytokine production via flow cytometry, and TCR sequencing.
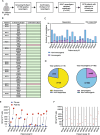
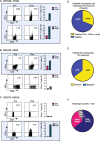
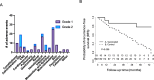
Results: Eighteen subjects received three doses of a neoantigen DNA vaccine encoding on average 11 neoantigens per patient (range 4-20). The vaccinations were well tolerated with relatively few adverse events. Neoantigen-specific T cell responses were induced in 14/18 patients as measured by ELISpot and flow cytometry. At a median follow-up of 36 months, recurrence-free survival was 87.5% (95% CI: 72.7-100%) in the cohort of vaccinated patients.
Conclusion: Our study demonstrates neoantigen DNA vaccines are safe, feasible, and capable of inducing neoantigen-specific immune responses.
Clinical trial registration number: NCT02348320.
Keywords: Clinical trial; DNA Vaccine; Immune response; Neoantigen; Phase I; TNBC.
© 2024. The Author(s).
Conflict of interest statement
Figures


References
-
- Matsushita H, Vesely MD, Koboldt DC, Rickert CG, Uppaluri R, Magrini VJ, et al. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature. 2012;482(7385):400–4. http://www.ncbi.nlm.nih.gov/pubmed/22318521:10.1038/nature10755. - DOI - PMC - PubMed
-
- Alspach E, Lussier DM, Miceli AP, Kizhvatov I, DuPage M, Luoma AM, et al. MHC-II neoantigens shape tumour immunity and response to immunotherapy. Nature. 2019;574(7780):696–701. https://www.ncbi.nlm.nih.gov/pubmed/31645760:10.1038/s41586-019-1671-8. - DOI - PMC - PubMed
-
- Zhang X, Kim S, Hundal J, Herndon JM, Li S, Petti AA, et al. Breast Cancer Neoantigens Can Induce CD8(+) T-Cell Responses and Antitumor Immunity. Cancer Immunol Res. 2017;5(7):516–23. https://www.ncbi.nlm.nih.gov/pubmed/28619968:10.1158/2326-6066.CIR-16-0264. - DOI - PMC - PubMed
-
- Li L, Zhang X, Wang X, Kim SW, Herndon JM, Becker-Hapak MK, et al. Optimized polyepitope neoantigen DNA vaccines elicit neoantigen-specific immune responses in preclinical models and in clinical translation. Genome Med. 2021;13(1):56. https://www.ncbi.nlm.nih.gov/pubmed/33879241:10.1186/s13073-021-00872-4. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

