Natural killer cell biology and therapy in multiple myeloma: challenges and opportunities
- PMID: 39538354
- PMCID: PMC11562869
- DOI: 10.1186/s40164-024-00578-4
Natural killer cell biology and therapy in multiple myeloma: challenges and opportunities
Abstract
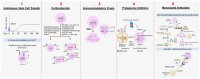
Despite therapeutic advancements, multiple myeloma (MM) remains incurable. NK cells have emerged as a promising option for the treatment of MM. NK cells are heterogenous and typically classified based on the relative expression of their surface markers (e.g., CD56 and CD16a). These cells elicit an antitumor response in the presence of low mutational burden and without neoantigen presentation via germline-encoded activating and inhibitory receptors that identify the markers of transformation present on the MM cells. Higher NK cell activity is associated with improved survival and prognosis, whereas lower activity is associated with advanced clinical stage and disease progression in MM. Moreover, not all NK cell phenotypes contribute equally toward the anti-MM effect; higher proportions of certain NK cell phenotypes result in better outcomes. In MM, the proportion, phenotype, and function of NK cells are drastically varied between different disease stages; this is further influenced by the bone marrow microenvironment, proportion of activating and inhibitory receptors on NK cells, expression of homing receptors, and bone marrow hypoxia. Antimyeloma therapies, such as autologous stem cell transplant, immunomodulation, proteasome inhibition, and checkpoint inhibition, further modulate the NK cell landscape in the patients. Thus, NK cells can naturally work in tandem with anti-MM therapies and be strategically modulated for improved anti-MM effect. This review article describes immunotypic and phenotypic differences in NK cells along with the functional changes in homeostatic and malignant states and provides expert insights on strategies to harness the potential of NK cells for improving outcomes in MM.
Keywords: Immunomodulation; Multiple myeloma; Natural killer cell activating receptor; Natural killer cell engagers; Natural killer cell inhibitory receptor; Natural killer cells.
© 2024. The Author(s).
Conflict of interest statement
Figures

References
-
- Kumar SK, Rajkumar V, Kyle RA, van Duin M, Sonneveld P, Mateos M-V, et al. Multiple myeloma. Nat Reviews Disease Primers. 2017;3(1):17046. - PubMed
-
- Alici E, Sutlu T, Björkstrand B, Gilljam M, Stellan B, Nahi H, et al. Autologous antitumor activity by NK cells expanded from myeloma patients using GMP-compliant components. Blood. 2008;111(6):3155–62. - PubMed
-
- Lin P, Reyes Silva FC, Lin P, Gilbert AL, Acharya S, Nunez Cortes AK, et al. CD70 CAR NK cells in the treatment of multiple myeloma. Blood. 2023;142(Supplement 1):3463.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

